Bacterial Vaginosis Rx Drug Market Outlook:
Bacterial Vaginosis Rx Drug Market size was valued at USD 1.06 billion in 2025 and is expected to reach USD 2.09 billion by 2035, expanding at around 7% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of bacterial vaginosis rx drug is evaluated at USD 1.13 billion.
The bacterial vaginosis Rx drug market is undergoing effective development, attributed to the rising incidence and increased awareness among the women population regarding the disorder. According to a report published by the Centers for Disease Control and Prevention (CDC), bacterial vaginosis affects almost 30% of women in the United States, accounting for an estimated 21 million women between the ages of 14 to 49 years. Therefore, this patient pool requires a suitable and strong supply chain-based active pharmaceutical ingredients (APIs), medical devices, and finished drug products, which positively drives the market demand globally.
Furthermore, the aspect of producer price index (PPI) and the consumer price index (CPI) are other factors readily driving the bacterial vaginosis Rx drug market internationally. The PPI for pharmaceuticals includes the overall cost trends for drug manufacturing. For instance, the Bureau of Labor Statistics (BLS) reported that the pharmaceutical preparations PPI surged by 2.8% between 2020 and 2021. Besides, the CPI for prescription-based medications has been increasing due to which consumer expenditure has also enhanced. For instance, the BLS also proclaimed that the prescription drug CPI has boosted by 1.9% during the same timeline.
Key Bacterial Vaginosis Rx Drug Market Insights Summary:
Regional Highlights:
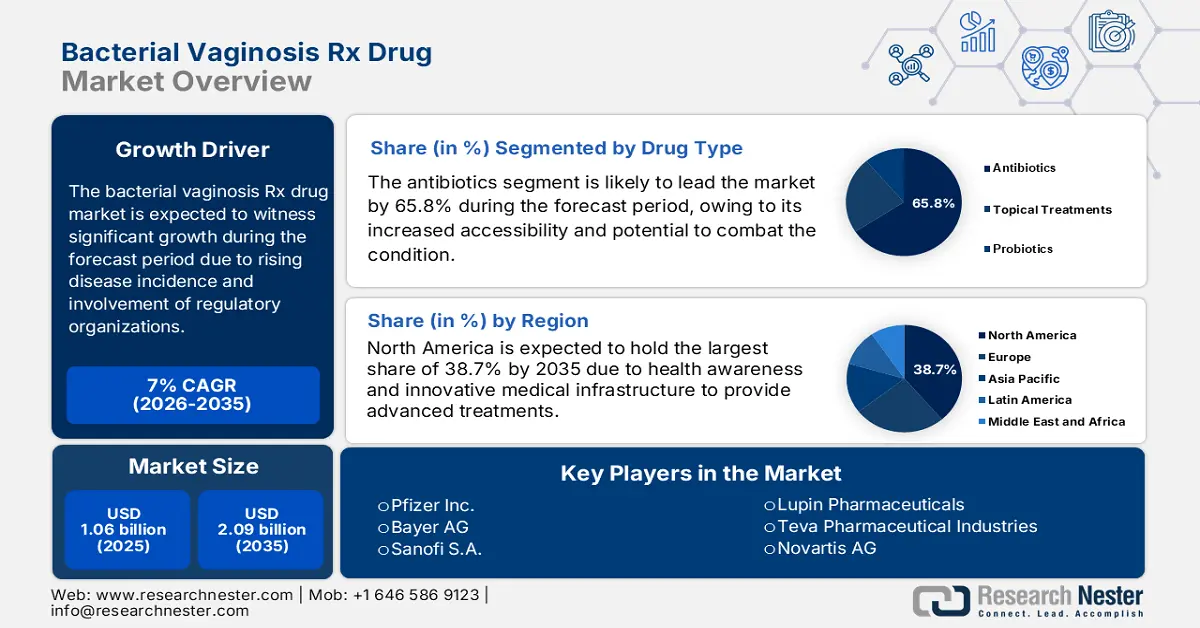
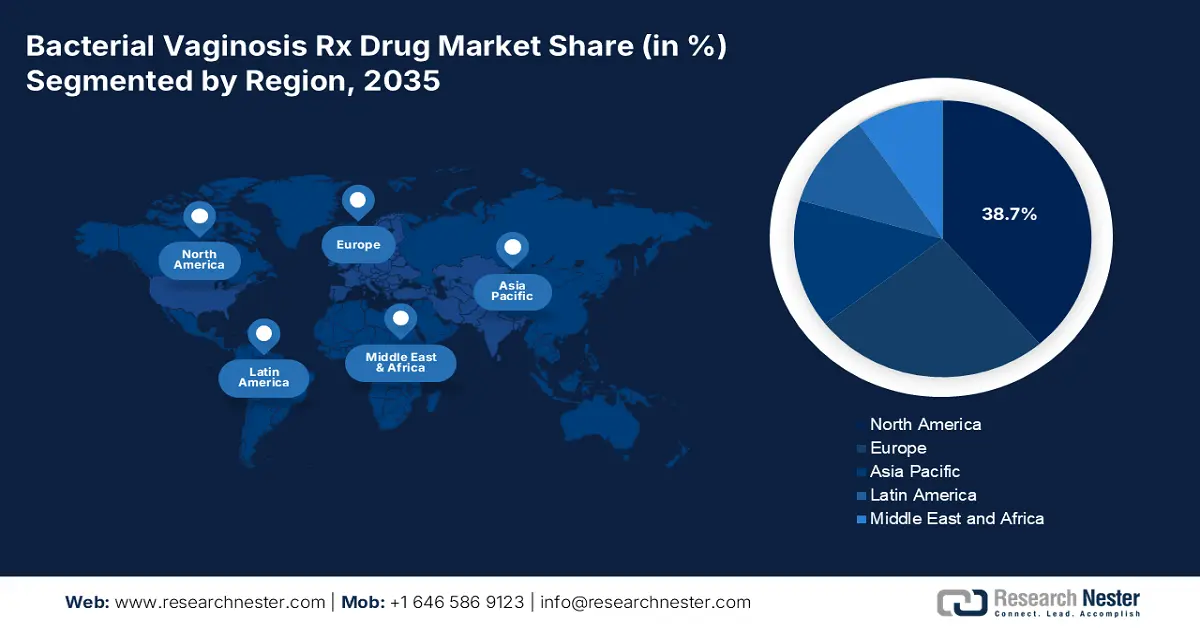
- North America leads the Bacterial Vaginosis Rx Drug Market with a 38.7% share, driven by growing awareness of women’s health and advanced medical infrastructure, ensuring sustained growth through 2026–2035.
- Europe is anticipated to hold a substantial share in the Bacterial Vaginosis Rx Drug Market from 2026 to 2035, driven by increased diagnosis accessibility, generic drug availability, and regulatory support.
Segment Insights:
- The Antibiotics segment is forecasted to capture a 65.8% share by 2035, propelled by the increased availability of effective antibiotics like clindamycin and metronidazole.
- The Hospital Pharmacies segment is expected to capture a 50.6% share by 2035 in the Bacterial Vaginosis Rx Drug market, propelled by the centralized healthcare model in regions like Europe and North America.
Key Growth Trends:
- Increased disease prevalence
- Administrative support and market enlargement
Major Challenges:
- Constraints in pricing and reimbursement policies
- Restricted market access
Key Players: Pfizer Inc., Bayer AG, Sanofi S.A., Lupin Pharmaceuticals.
Global Bacterial Vaginosis Rx Drug Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 1.06 billion
- 2026 Market Size: USD 1.13 billion
- Projected Market Size: USD 2.09 billion by 2035
- Growth Forecasts: 7% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (38.7% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, United Kingdom, France, Japan
- Emerging Countries: China, India, Brazil, Mexico, Russia
Last updated on : 12 August, 2025
Bacterial Vaginosis Rx Drug Market Growth Drivers and Challenges:
Growth Drivers
-
Increased disease prevalence: Bacterial vaginosis is considered the most prevalent infection that occurs in the vaginal area which effectively impacts the health of women at their reproductive age. As per an article published by NLM, the occurrence rate of the condition usually ranges from 23% to 29%, which denotes a surge in the demand for the bacterial vaginosis Rx drug market across nations. Besides, in the United States, the financial burden of aiding symptomatic bacterial vaginosis is approximately USD 5.1 billion on a yearly basis, which includes additional costs arising from HIV cases and preterm births.
-
Administrative support and market enlargement: The presence and availability of regulatory organizations such as the U.S. Food and Drug Administration (FDA) have accepted the latest treatment solutions for the disease, facilitating the bacterial vaginosis Rx drug market expansion. For instance, in February 2022, Lupin Pharmaceuticals Inc. declared the U.S. FDA approval for its complementary New Drug Application (sNDA) to increase the utilization of SOLOSEC. This is suitable for treating more than 12-year-old female patients, thus driving the market globally.
Challenges
-
Constraints in pricing and reimbursement policies: The recurrence rate of the disease across nations has led to an upsurge in treatment costs, which negatively impacts the bacterial vaginosis Rx drug market internationally. In a clinical study published by NLM, it has been found that the average health spending is USD 5,974.5 per patient, with the bacterial vaginosis cost starting at USD 300 per patient. This has underscored the economic burden, especially on the Medicaid systems, thus limiting the reimbursement policies for the condition.
-
Restricted market access: There is a limited availability of resources and presence of healthcare infrastructure, especially in low- and middle-income nations, which denotes another challenging aspect for the bacterial vaginosis Rx drug market. As stated in an article published by the World Health Organization (WHO) in November 2024, an increase in research and developmental activities to gather evidence is an appropriate solution to combat restrictions and limitations to gain market access, as well as reduce the occurrence of the disease across nations.
Bacterial Vaginosis Rx Drug Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
7% |
|
Base Year Market Size (2025) |
USD 1.06 billion |
|
Forecast Year Market Size (2035) |
USD 2.09 billion |
|
Regional Scope |
|
Bacterial Vaginosis Rx Drug Market Segmentation:
Drug Type (Antibiotics, Topical Treatments, Probiotics)
Based on the drug type, the antibiotics segment is expected to hold the largest share of 65.8% in the bacterial vaginosis Rx drug market by the end of the forecast timeline. The segment’s growth is driven by its increased availability and potential to aid bacterial vaginosis by targeting the anaerobic bacteria, which is the actual cause of the condition. Clindamycin and metronidazole are the most commonly available and prescribed antibiotics for the treatment procedure, which effectively contributes towards the market development across nations.
Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, Clinics, Mail Order Pharmacies)
Based on the distribution channel, the hospital pharmacies segment is expected to account for a considerable share of 50.6% in the bacterial vaginosis Rx drug market by the end of 2035. The aspect of centralized healthcare nature in many geographic landscapes is the ultimate driving factor for the segment’s development, especially in Europe and North America. Besides, hospital as a healthcare facility is recognized as the point of care for bacterial vaginosis diagnosis and treatment since health providers usually conduct assessments for evaluation and accordingly prescribe ailment options within clinical environments, thus a positive outlook for the segment.
Our in-depth analysis of the global market includes the following segments:
|
Drug Type |
|
|
Distribution Channel |
|
|
Route of Administration |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Bacterial Vaginosis Rx Drug Market Regional Analysis:
North America Market Analysis
The North America region is anticipated to account for the highest share of 38.7% in the bacterial vaginosis Rx drug market during the forecast timeline. This growth is fueled by factors such as women's health awareness, increased healthcare expenditure, and cutting-edge medical infrastructure. In addition, the market in the region is also attributed to a robust presence of pharmaceutical organizations, continuous research for the latest drug products, and a well-developed distribution network.
The market in the U.S. is significantly growing due to the presence of governmental agencies and clinical trials conducted. For instance, according to an article published by CDC, the treatment procedure of the condition comprises 1.3% of single dose metronidazole vaginal gel and 2% of single dose clindamycin phosphate vaginal cream. In this regard, a Phase 3 clinical study was conducted, wherein the cure rate for metronidazole was 37.2% in comparison to 26.6% for placebo, thereby deriving an effective treatment solution in the region.
The market in Canada is steadily developing, supported by government investment to cater to women’s health. For instance, the Government of Canada initiated an investment of more than USD 1.7 million to extend support for projects put forward by the Sex Information and Education Council of Canada and the Society of Obstetricians and Gynaecologists of Canada. Additionally, the Society of Obstetricians and Gynaecologists of Canada is yet to receive USD 1.2 million for the upcoming three years to develop resources and tools for women's health, thus positively driving the market evolution.
Europe Regional Market Size & Growth
The Europe bacterial vaginosis Rx drug market is projected to hold the second-largest share of 25.8% by the end of the forecast period. Factors such as increased accessibility of diagnosis and treatment processes, generic medication availability, and advanced pharmaceutical formulations are readily responsible for the market upliftment in the region. Germany, France, and the U.K. are leading contributors to the regional market with sufficient investment-based strategies owing to high disease prevalence. Besides, the presence of administrative agencies such as the Europe Health Data Space and the Europe Medicines Agency (EMA) is inevitably involved in regulating and accepting treatment options.
The bacterial vaginosis Rx drug market in Germany is one of the largest markets in the region with an expenditure of €4 billion on healthcare as of 2024. The country comprises a robust pharmaceutical industry, which is the most prominent contributing factor to the market expansion. Besides, there is a surge in the incidence of vaginal infection in the country due to factors including inconsistent condom utilization, increased hormonal fluctuations, and numerous sexual partners, all of which is driving the market demand.
There is a huge opportunity for the bacterial vaginosis Rx drug market in France owing to enhanced focus on healthcare initiatives and promoting healthy lifestyles. This has been possible through an allocation of 7.3% of its healthcare funding to commercialize bacterial vaginosis treatments since 2023. Besides, the French Ministry of Solidarity and Health has promoted treatment coverage strategies under the national health insurance scheme. This action plan has enhanced prescription drugs, which eventually led to an increase in the patient base, thereby suitable for market expansion in the country.
Key Bacterial Vaginosis Rx Drug Market Players:
- Pfizer Inc. (U.S.)
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Bayer AG
- Sanofi S.A.
- Lupin Pharmaceuticals
- Teva Pharmaceutical Industries
- Novartis AG
- Mylan N.V.
- Perrigo Company plc
- Starpharma Holdings Limited
- Glenmark Pharmaceuticals Ltd
The bacterial vaginosis Rx drug market upliftment is characterized by diversified international organizations and manufacturers, each engaged in implementing exclusive approaches to boost their market presence. In addition, they are focusing on developing innovative therapies that include combination treatment options for patients and providers to integrate antibiotics with probiotics. This has proven to be useful and has been successfully implemented by Sanofi S.A., Lupin Pharmaceuticals, Teva Pharmaceutical Industries, Starpharma Holdings Limited, and Mission Pharmacal, thus denoting a positive influence on the market globally.
Here is a list of key players operating in the global market:
Recent Developments
- In April 2024, Pfizer Inc. declared positive results from clinical trials for an oral medication for bacterial vaginosis, leading to the company expanding its portfolio in women's health and addressing their unmet needs.
- In June 2022, Duchesnay Inc. launched Vablys, which is the first prescription antiseptic and anti-infective treatment for bacterial vaginosis, especially manufactured for women under 55 years of age.
- Report ID: 7664
- Published Date: Aug 12, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Bacterial Vaginosis Rx Drug Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




