S1P Receptor Modulator Drugs Market Outlook:
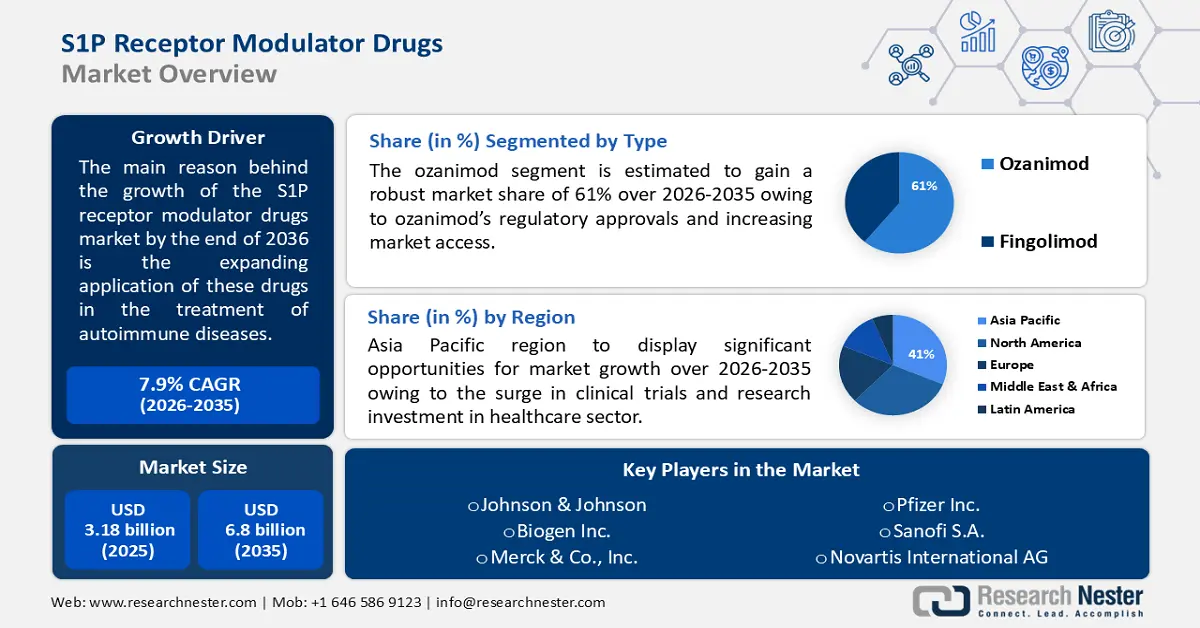
S1P Receptor Modulator Drugs Market size was valued at USD 3.18 billion in 2025 and is expected to reach USD 6.8 billion by 2035, expanding at around 7.9% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of S1P receptor modulator drugs is evaluated at USD 3.41 billion.
The S1P receptor modulator drugs market is experiencing growth, which is primarily driven by the expanding applications of these drugs in the efficient treatment of autoimmune diseases. S1P receptors are integral to the functioning of the immune system by playing a crucial role in the modulation of lymphocyte trafficking.
By targeting these receptors, S1P receptor modulator drugs can have an extreme impact on the immune response and make them effective candidates for treating autoimmune disorders.
As autoimmune diseases continue to pose significant health challenges globally, the demand for innovative and targeted therapeutic solutions has grown exponentially. According to a report published in February 2024, around 5-10% of the global population is diagnosed with autoimmune diseases.
S1P receptor modulators are a class of drugs that target receptors for sphingosine-1-phosphate, a lipid signaling. Further, these receptors play a crucial role in regulating the immune system, and modulating them can have therapeutic effects on autoimmune diseases. Additionally, these are the other factors responsible for market expansion.
Key S1P Receptor Modulator Drugs Market Insights Summary:
Regional Highlights:
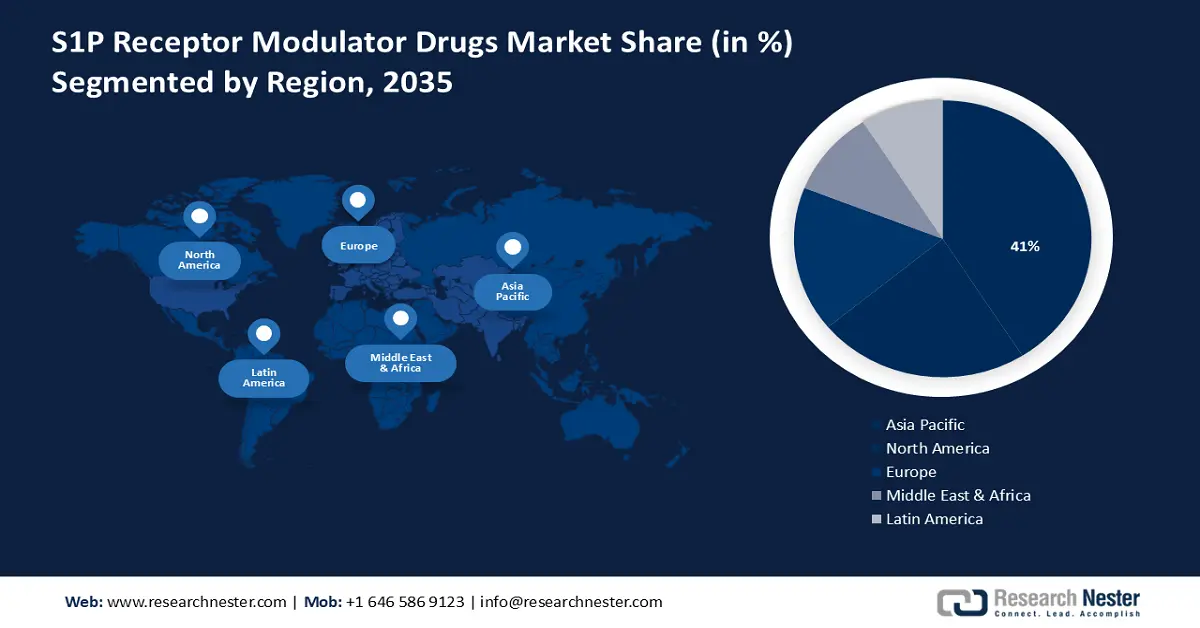
- By 2035, the Asia Pacific region is anticipated to secure a dominant 41% share in the S1P receptor modulator drugs market, attributed to the surge in clinical trials and rising research investments.
- The North American region is expected to capture a substantial share by 2035, underpinned by the high prevalence of multiple sclerosis and autoimmune disorders.
Segment Insights:
- By 2035, the ozanimod segment in the S1P receptor modulator drugs market is projected to command around 61% share, propelled by regulatory approvals and expanding market access.
- The hospital segment is set to attain a notable share by 2035, supported by the growing emphasis on value-based healthcare models.
Key Growth Trends:
- Expanding applications in autoimmune diseases
- Rising investment in research and development (R&D)
Major Challenges:
- Safety concerns and adverse effects
- High development costs and pricing pressures
Key Players: Novartis International AG, Bristol Myers Squibb Company, Johnson & Johnson, Biogen Inc., Merck & Co., Inc., F. Hoffmann-La Roche Ltd.
Global S1P Receptor Modulator Drugs Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 3.18 billion
- 2026 Market Size: USD 3.41 billion
- Projected Market Size: USD 6.8 billion by 2035
- Growth Forecasts: 7.9%
Key Regional Dynamics:
- Largest Region: Asia Pacific (41% Share by 2035)
- Fastest Growing Region: North America
- Dominating Countries: United States, China, Japan, Germany, United Kingdom
- Emerging Countries: India, South Korea, Brazil, Australia, Canada
Last updated on : 27 November, 2025
S1P Receptor Modulator Drugs Market - Growth Drivers and Challenges
Growth Drivers
- Expanding applications in autoimmune diseases - The S1P receptor modulator drugs market is witnessing substantial growth, primarily propelled by the expanding applications of these drugs in treating autoimmune diseases.
S1P receptor modulation has proven efficacious in immune system regulation, making these drugs promising candidates for a broad spectrum of autoimmune conditions. Autoimmune diseases affect millions worldwide, and the demand for targeted therapies is on the rise.
Ongoing clinical trials research help to explore the application in rheumatoid arthritis, lupus, and inflammatory bowel diseases, highlighting the versatility of S1P receptor modulators. - Rising investment in research and development (R&D)-The S1P receptor modulator drugs market is flourishing due to a surge in investment in research and development (R&D). Pharmaceutical companies are increasingly allocating resources to explore novel applications, formulations, and combinations of S1P receptor modulators, driving innovation and market growth. According to a recent analysis, global pharmaceutical R&D spending reached USD 243 billion in 2022.
As the pharmaceutical landscape evolves, there is a heightened focus on developing breakthrough therapies. S1P receptor modulators, being at the intersection of immunology and neurology, attract substantial R&D investments. - Expanding geographical presence and market access - The S1P receptor modulator drugs market is expanding its geographical presence and market access, fostering growth through increased availability and accessibility of these innovative therapies.
Efforts to secure regulatory approvals in diverse regions and enhance market penetration contribute significantly to the overall market expansion. The global reach of the S1P receptor modulator drugs market is expanding as regulatory agencies worldwide approve these drugs for various indications.
Companies are strategically navigating regulatory landscapes to secure approvals in multiple regions, ensuring a broader patient population can access these therapies.
Challenges
- Safety concerns and adverse effects - As with any pharmaceutical intervention, the safety profile of S1P receptor modulator drugs is a critical concern. Adverse effects, including cardiovascular events and macular edema, have been associated with certain medications in this class. Balancing therapeutic efficacy with potential risks poses a challenge in the clinical application of these drugs.
The research and development costs associated with bringing new drugs to market are substantial. Developing S1P receptor modulator drugs involves intricate research, extensive clinical trials, and regulatory processes. High development costs, coupled with increasing pricing pressures and healthcare budget constraints, pose challenges for market access and affordability. - High development costs and pricing pressures
- Competitive landscape and generic erosion
S1P Receptor Modulator Drugs Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
7.9% |
|
Base Year Market Size (2025) |
USD 3.18 billion |
|
Forecast Year Market Size (2035) |
USD 6.8 billion |
|
Regional Scope |
|
S1P Receptor Modulator Drugs Market Segmentation:
Type Segment Analysis
In S1P receptor modulator drugs market, ozanimod segment is likely to capture around 61% share by the end of 2035. Ozanimod's regulatory approvals and increasing market access contribute significantly to its growth. Regulatory clearances enhance the drug's availability, making it accessible to a broader patient population, thereby influencing its market impact.
Ozanimod received regulatory approvals from major health authorities, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), underscoring its safety and efficacy. These approvals facilitate market access, allowing healthcare providers to prescribe Ozanimod as part of their treatment.
Ozanimod capsules received FDA approval in March 2020 for the treatment of relapsing forms of MS. These regulatory milestones signify Ozanimod's compliance with rigorous standards, contributing to its acceptance as a therapeutic option in the MS sector.
End User Segment Analysis
The hospital segment in the S1P receptor modulator drugs market is expected to garner a significant share by 2035. The growing emphasis on value-based healthcare models is a significant driver for the adoption of S1P receptor modulator drugs in hospitals. Value-based care focuses on improving patient outcomes while optimizing healthcare costs, aligning with the goals of healthcare providers in hospital settings.
The shift towards value-based care creates opportunities for hospitals to adopt advanced therapies, including S1P receptor modulator drugs, that align with these models. S1P receptor modulator drugs, by offering targeted and effective treatments for autoimmune diseases, contribute to value-based care objectives.
Hospitals, recognizing the potential to enhance patient outcomes and reduce the economic burden associated with autoimmune diseases, are inclined to integrate these innovative therapies into their care models.
Our in-depth analysis of the global S1P receptor modulator drugs market includes the following segments:
|
Type |
|
|
End User |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
S1P Receptor Modulator Drugs Market - Regional Analysis
APAC Market Insights
Asia Pacific industry is predicted to dominate majority revenue share of 41% by 2035. The surge in clinical trials and research investment in the healthcare sector is a noteworthy driver for the market. As the region becomes a focal point for pharmaceutical research, the momentum in clinical investigations contributes to the expansion of treatment options.
Pharmaceutical companies are increasingly conducting clinical trials in the Asia Pacific region to tap into diverse patient populations and benefit from the region's growing research capabilities. The presence of clinical trial sites and investment in research infrastructure create an environment conducive to the exploration of S1P receptor modulator drugs for various autoimmune indications.
The growth of the S1P receptor modulator drugs market in the Asia Pacific region is further propelled by the increasing prevalence of autoimmune diseases, rising healthcare expenditure, government initiatives, patient awareness efforts, collaborations in clinical research, and the surge in clinical trials and research investment. In India, the investments in R&D reached USD 17.2 billion as per the R&D statistics (2022-2023) of the Department of Science and Technology.
North American Market Insights
The S1P receptor modulator drugs market in the North American region is projected to hold a significant share during the forecast period. The high prevalence of multiple sclerosis and autoimmune diseases in North America is a fundamental driver for the growth of the market.
The region has a significant burden of autoimmune conditions, creating a robust demand for innovative and targeted therapies. North America, particularly the United States and Canada, has reported a substantial incidence of multiple sclerosis and various autoimmune diseases.
According to a report, nearly 1 million people are diagnosed with MS in the United States. The prevalence of autoimmune diseases in North America, with millions of affected individuals, underscores the significant patient population that can benefit from S1P receptor modulator drugs.
The favorable regulatory environment in North America, characterized by efficient review processes and fast-track approvals, is a crucial growth driver for the S1P receptor modulator drugs market. Regulatory agencies in the region prioritize innovation, enabling swift market access for novel therapies.
S1P Receptor Modulator Drugs Market Players:
- Novartis International AG
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Bristol Myers Squibb Company
- Johnson & Johnson
- Biogen Inc.
- Merck & Co., Inc.
- F. Hoffmann-La Roche Ltd.
- Quality Pharma Products Pvt. Ltd.
- Pfizer Inc.
- Sanofi S.A.
- Medicinum Healthcare Pvt. Ltd.
Recent Developments
- Bristol Myers Squibb announced a definitive agreement to acquire Mirati Therapeutics, a commercial-stage targeted oncology company specializing in KRAS G12C inhibitors. This USD 4.8 billion acquisition adds the FDA-approved KRAZATI® (adagrasib) to BMS's portfolio, along with promising early-stage assets targeting KRAS and other driver mutations.
- BMS and Seagen announced a collaboration to launch a Phase 3 trial evaluating ADCETRIS® in combination with chemotherapy for treatment-naive adult patients with Hodgkin lymphoma. This partnership leverages the strengths of both companies to pursue potentially curative options for this cancer.
- Report ID: 5529
- Published Date: Nov 27, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
S1P Receptor Modulator Drugs Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




