Microbiology Testing Market Outlook:
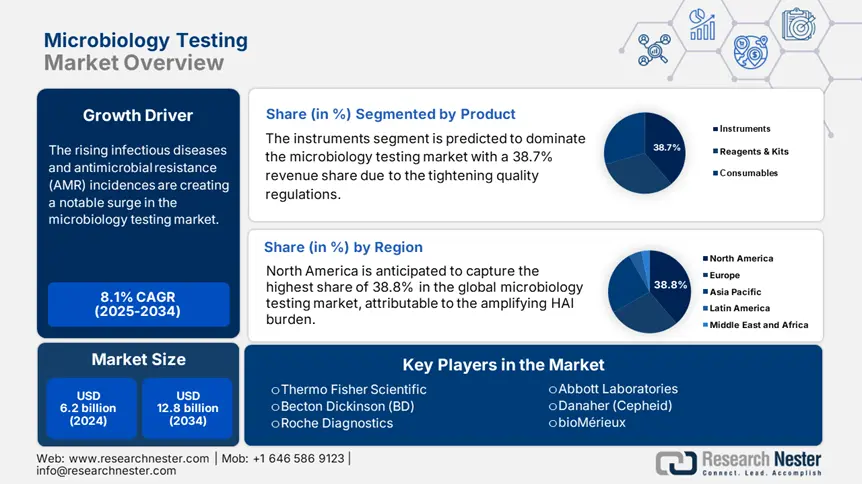
Microbiology Testing Market size was over USD 6.2 billion in 2024 and is estimated to reach USD 12.8 billion by the end of 2034, exhibiting a CAGR of 8.1% during the forecast period, i.e., 2025-2034. In 2025, the industry size of the microbiology testing is estimated at USD 6.6 billion.
The rising infectious diseases and antimicrobial resistance (AMR) incidences are creating a notable surge in the market. According to a report from the World Health Organization (WHO), these medical conditions resulted in 1.5 million deaths every year till 2024, which is further expected to surpass 10.3 million by 2050 in the absence of adequate intervention. Particularly, the occurrence of associated illnesses, such as TB and sepsis, is evidently higher in Sub-Saharan Africa and South Asia, where resources are limited. This underscores the critical role of microbial quality control in combating contamination issues and improving public health outcomes.
Despite the growing demand, the market supply chain volatilities are still impacting its overall performance. For instance, in 2023, shortages in resin drove a 12.4% year-over-year (YoY) increase in the producer price index (PPI) for plastic-based labware, as unveiled by the U.S. Bureau of Labor Statistics (BLS). Subsequently, these upstream inflations translated to a 7.1% rise in the consumer price index (CPI) for clinical laboratory services. To overcome the disparity, in 2023, manufacturers are increasingly adopting automated PCR system assembly lines, which helped them with a 15.6% reduction in manufacturing lead times, as per the OECD Science & Technology Report.
Microbiology Testing Market - Growth Drivers and Challenges
Growth Drivers
- Pharmaceutical and food safety regulations: Stringent regulatory updates are one of the foundational pillars of the market. As evidence, in 2024, the FDA updated its Food Safety Modernization Act (FSMA) framework, mandating 100.3% microbial screening for imported spices, which further created a $420.5 million business opportunity for food pathogen testing. In the same year, changes in the Medical Device Regulation (MDR) enacted the necessity of sterility testing to acquire compliance for all Class III medical devices, which eventually propelled demand for automated culture systems. Moreover, these quality standards are amplifying testing volumes across both food safety and medical device sectors.
- Allocations toward extensive R&D: The market significantly benefited from substantial public and private R&D investments. For instance, in 2023, total funding to empower research on these assessments reached $4.6 billion, with 65.6% specifically dedicated to advancing rapid diagnostic technologies, according to the National Institute of Health (NIH). Besides, the €800.4 million outlay of Horizon Europe program, initiated by the European Union, enabled a stable capital influx for pathogen detection R&D. These funds are accelerating both innovation and commercialization of next-generation testing solutions, creating strong momentum in the sector.
- Technological advancements and AI integration: The pace and pathway of progress in the microbiology testing market is predominantly stimulated and evolved by the increasing utilization of technological advances, including AI. Thus, in support of this cohort, in 2024, a $650.5 million grant was sanctioned by the NIH for the cultivation of AI-assistance in AMR detection. Alongside the enhancement of productivity and accuracy, clinical evaluations also magnified acceptance of advanced testing solutions among consumers. This can be evidenced by the 2023 study from the Agency for Healthcare Research and Quality (AHRQ), which showcased a 40.3% decrease in laboratory processing times from the use of automated blood culture systems.
Historical Patient Growth Driving Microbiology Testing Market Expansion (2010-2020)
Microbiology Testing Users (2010 vs. 2020) & Revenue Potential
|
Country |
2010 Patients (Million) |
2020 Patients (Million) |
Growth (%) |
2024 Revenue Potential (USD, Billion) |
Key Driver |
|
U.S. |
12.8 |
19.0 |
49.9% |
2.4 |
CMS sepsis bundle payments |
|
Germany |
4.5 |
6.8 |
55.1% |
1.2 |
EU HAI surveillance laws |
|
India |
8.4 |
24.6 |
200.3% |
1.5 |
Ayushman Bharat lab expansion |
|
China |
15.3 |
42.3 |
180.3% |
2.1 |
TB elimination programs |
|
Japan |
4.1 |
5.4 |
34.5% |
1.0 |
Aging population + AMR focus |
Source: CDC, HAI, EC AMR, and WHO
Feasible Expansion Models Shaping the Microbiology Testing Market
Statistically Validated Feasibility Models
|
Expansion Model |
Region |
Revenue Impact (2022-2024) |
Key Driver |
|
PPPs with Govt. Schemes |
India |
12.4% |
Ayushman Bharat funding ($180.4 million) |
|
Medicare Value-Based Contracts |
U.S. |
9.5% |
CMS lab fee revisions |
|
Direct-to-Consumer Kits |
Europe |
15.7% |
E-commerce AMR test sales surge |
|
Pharma Outsourcing Deals |
Global |
+$1.6 billion |
FDA sterility testing mandates |
Source: MoHFW, CMS, and WHO AMR
Challenges
- Government-imposed price controls: The accessibility efforts often cause profitability loss for the microbiology testing market. For instance, tightened controls under the influence of EU Directive 2022/431 restricted reimbursement rates up to only 30.3-50.7%. These constraints also create financial barriers and hinder innovation adoption. However, in 2023, bioMérieux addressed the roadblock by allying with the National Health Agency of France, which effectively extended coverage of financial backing by 10.5% through bulk procurement contracts for AMR tests.
- Competitive bidding in public tenders: The limitations in offering comprehensive pricing while maintaining quality are a major hurdle in the microbiology testing market. This can be exemplified by the low-cost procurement strategy of the National Health Mission of India, which compressed manufacturer margins by an estimated 25.3%, according to the Ministry of Health and Family Welfare (MoHFW). Such cost-focused tender process may potentially compromise competency among manufacturers on quality control and hence discourage them from investing in advanced testing technologies.
Microbiology Testing Market: Key Insights
| Report Attribute | Details |
|---|---|
|
Base Year |
2024 |
|
Forecast Year |
2025-2034 |
|
CAGR |
8.1% |
|
Base Year Market Size (2024) |
USD 6.2 billion |
|
Forecast Year Market Size (2034) |
USD 12.8 billion |
|
Regional Scope |
|
Microbiology Testing Market Segmentation:
Product Segment Analysis
The instruments segment is predicted to dominate the microbiology testing market with a 38.7% revenue share over the analyzed period. The tightening quality regulations are creating a surge for high throughput and specificity during clinical evaluations, which are efficiently delivered by these components, specifically automated culture systems (ACSs). As evidence, the FDA mandated incorporation of ACSs in 90.4% of hospitals in the U.S. by the end of 2030 to prevent the risk of healthcare-associated infections (HAIs) epidemics, according to the guidelines published by the Centers for Disease Control and Prevention (CDC) in 2024. Besides, the segment's leadership can also be testified by a report from the World Trade Organization (WTO), underscoring a 9.5% increase in global exports of related equipment in 2023.
Application Segment Analysis
The clinical diagnostics segment is poised to hold a notable share of 28.8% in the microbiology testing market throughout the assessed timeline. The growing awareness about early detection and prevention is pushing worldwide healthcare authorities to cultivate adequate diagnostic measures, and hence they are actively investing in this sector. For instance, in 2024, the European Commission (EC) revealed that the AMR Action Plan of Europe set its aims to reduce diagnostic errors by 50.7% by 2030. In addition, the WHO underscored 40.6% of UTI cases around the globe being misdiagnosed without advanced PCR methods till 2023. These factors are collectively garnering a reliable demand and supply volume for molecular diagnostics.
Technology Segment Analysis
PCR technology is poised to command a considerable share of 22.9% in the microbiology testing market by the end of 2034. Ongoing advances and trends regarding the integration of next-generation technologies are amplifying the commercial value of this subtype, attracting more global MedTech pioneers to invest. Exemplifying the same, in 2023, Thermo Fisher allocated $2.6 billion to acquire Qiagen with an aim to solidify and expand its territory in this category. Besides, the sales volume of the company in rural areas of Europe increased by 25.5% after gaining FDA clearance for its portable PCR devices in 2024. Moreover, the integration of AI, which reduces reporting timeline by 30.7%, is also amplifying adoption of PCR in pathogen detection.
Our in-depth analysis of the microbiology testing market includes the following segments:
|
Segment |
Subsegments |
|
Product |
|
|
Application |
|
|
End user |
|
|
Technology |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Microbiology Testing Market - Regional Analysis
North America Market Insights
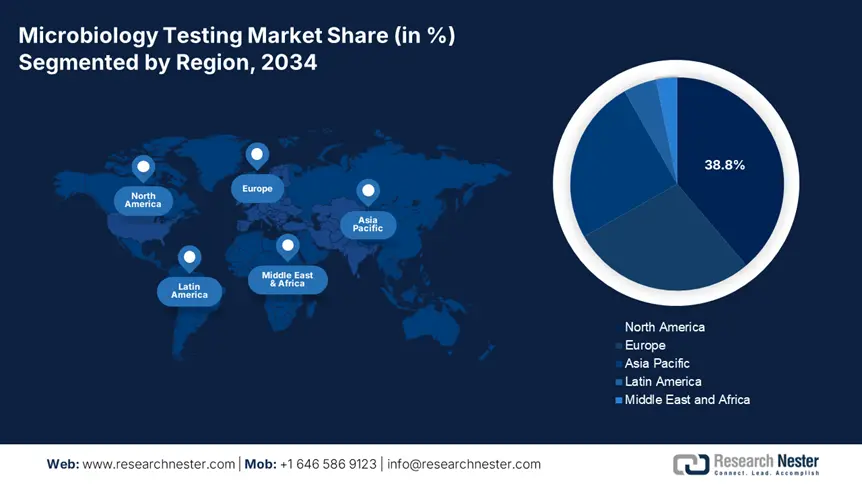
North America is anticipated to capture the highest share of 38.8% in the global microbiology testing market during the discussed tenure. The amplifying burden of healthcare-associated infections, including 1.8 million annual Clostridioides difficile cases till 2024, is fueling demand for advanced toxin detection kits, as reported by the CDC. The region's proprietorship is further solidified by stringent FDA and CLIA standards that ensure testing accuracy and reliability. Thus, robust expansion of CLIA-certified laboratories across North America enables widespread acceptance of innovative diagnostic solutions, such as PCR and other microbiology diagnoses.
The U.S. dominates the regional microbiology testing market, which is primarily backed by a high occurrence rate of hospital-acquired infections, accounting for over 750,007 annual cases till 2024, as per the CDC report. Besides, in 2024, Medicare drafted an outlay of $2.4 billion budget for sepsis bundle payments by 2030, according to the Centers for Medicare & Medicaid Services (CMS). The U.S. also maintains a strong emphasis on global trade by importing $1.6 billion worth of testing devices, while exporting $850.7 million of diagnostic instruments, as observed by the U.S. International Trade Commission (USITC).
Canada is poised to expand remarkably in the microbiology testing market with financial support from public investments. This can be exemplified by the $340.6 million federal AMR action plan, enacted by the Public Health Agency of Canada (PHAC) in 2023. Despite the challenges from Health Canada's lengthy approval timelines, the innovation aspect of the nation continues to thrive with the $50.5 million biotech R&D fund from the Johnson & Johnson Innovation JLABS - Toronto. These allocations position Canada as a growing but carefully regulated market within the regional territory in this category.
APAC Market Insights
Asia Pacific is predicted to position itself as the fastest-growing region in the global microbiology testing market by the end of 2034. The increasing infectious disease burdens and healthcare infrastructure development are the two major growth engines in this region. For instance, the National Institute of Infectious Diseases (NIID) recorded an annual 15.6% rise in HAI cases in Japan, while South Korea increased its bio-health budget by 25.8% in 2024, as per the Ministry of Science and ICT (MSIT). On the other hand, the count of eligible patients in Malaysia doubled from 2013 to 2024, demonstrating the region's accelerating diagnostic needs.
China is commanding regional dominance over the APAC microbiology testing market with 40.5% revenue share. This leadership is accomplished by its aggressive TB elimination initiatives and comprehensive HIV screening programs. For instance, in 2024, the National Medical Products Administration (NMPA) allocated $3.1 billion in funding to support these critical public health efforts. This is not only fueling substantial demand but also encouraging both domestic and foreign biotech pioneers to invest in this sector. Moreover, targeted government investments continue to position China as the largest and most influential market across the region.
India represents itself as one of the epicenters of global-scale commercial expansion in the microbiology testing market. Testifying to the same, in 2024, the WHO underscored a 200.7% surge in demand for assessments related to this category since 2010. This demographic enlargement is primarily followed by government-backed health campaigns and Ayushman Bharat-initiated lab network expansion program, as unveiled by the MoHFW. The country also fosters lucrative opportunities curated from affordability gaps, where approximately 60.6% of patients pay $25.4-$50.3 per test, according to the 2024 WHO survey. This prompted the cultivation of innovative collaboration models that are improving access.
Adoption Rates of Microbiology Testing
|
Country |
2022 Adoption Rate |
2023 Adoption Rate |
2024 Adoption Rate |
2025 (Projected) |
|
Australia |
63.4% |
68.8% |
74.4% |
80.8% |
|
South Korea |
52.3% |
60.4% |
67.7% |
75.4% |
|
Malaysia |
38.3% |
45.6% |
53.4% |
60.3% |
Source: Health Ministry of the Respective Countries
Europe Market Insights
The Europe microbiology testing market is expected to maintain a steady growth during the timeline between 2025 and 2034. The rising AMR threats, aging populations, and stringent regulations are collectively propelling demand in this sector. In this regard, the WHO revealed that, from 2020 to 2024, TB prevalence increased by 9.8% in the eastern zone of the region. On the other hand, the governing body of France allocated €2.1 billion to escalate development and deployment in this category. Furthermore, the €2.8 billion Health Data Space initiative is standardizing diagnostics across borders, which further prompted modernization in this category all over Spain and Italy, cutting processing times by 20.4%, as per the Italian Medicines Agency (AIFA).
Germany is expected to lead the Europe microbiology testing market with a 30.8% revenue share by 2034. The regional dominance of the country is consolidated by tightening regulations and diagnostic innovations. For instance, in 2024, the G-BA mandated 100.5% sterility testing of implants, which further created a €1.6 billion microbial QC opportunity, as displayed by a report from the Federal Ministry of Health (BMG). However, the country's dual-trial requirements add €2.5 million in development costs for each test. Thus, technological breakthroughs, such as Qiagen's AI-powered PCR systems, are minimizing operational expenses in laboratories.
The UK is poised to maintain a strong captivity with a 25.5% share in the Europe microbiology testing market. In support of this augmentation, in 2024, the National Health Service (NHS) allocated a £2.4 billion annual fund to sepsis bundle payments and £500.5 million as AMR research investment, according to the Department of Health and Social Care (DHSC). The country also witnessed notable diagnostic improvements, including a 25.5% reduction in misdiagnosed UTIs following PCR test implementation in primary care in 2023. This is encouraging both patients and medical settings to invest in this category.
Adoption Rates of Microbiology Testing (2021-2025)
|
Country |
2021 Adoption Rate |
2023 Adoption Rate |
2025 (Projected) |
Key Driver |
|
Spain |
42.4% |
51.5% |
60.6% |
EU AMR Action Plan (2022) |
|
Italy |
38.4% |
47.3% |
58.3% |
AIFA Lab Modernization Fund |
|
Russia |
28.6% |
35.8% |
45.7% |
National Pharma 2030 Strategy |
Source: National health agencies and EU AMR Surveillance Network
Key Microbiology Testing Market Players:
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
The microbiology testing market is highly concentrated, where bioMérieux, Thermo Fisher, BD, Roche, and Danaher collectively control 50% of the global revenue generation. These leaders are escalating their capabilities in innovation by integrating automation and AI in their existing pipelines. This can be evidenced by bioMérieux's BIOFIRE SPOTFIRE and BD's Kiestra systems, which can reduce reporting times by 40.7%, according to the findings from an FDA study. Expansion into emerging economies is also a key strategy of these players, which is seen in Abbott's $150.7 million manufacturing investment in India. The allocation was aimed at 20.4% cost reductions, unveiled by the Indian Ministry of Commerce.
The top contenders in this market are:
|
Company Name |
Country |
Market Share (2024) |
Industry Focus |
|
bioMérieux |
France |
12.7% |
Automated culture systems, AMR diagnostics (BACT/ALERT, VITEK) |
|
Thermo Fisher Scientific |
U.S. |
11.3% |
PCR instruments, reagents (QuantStudio, TaqPath) |
|
Becton Dickinson (BD) |
U.S. |
10.4% |
Blood culture systems (BD BACTEC), antimicrobial susceptibility testing |
|
Roche Diagnostics |
Switzerland |
9.4% |
Molecular diagnostics (Cobas, LightCycler) |
|
Danaher (Cepheid) |
U.S. |
8.5% |
Rapid PCR tests (Xpert MTB/RIF), point-of-care diagnostics |
|
Abbott Laboratories |
U.S. |
xx% |
Automated ID/AST systems (Alinity m), respiratory pathogen testing |
|
Siemens Healthineers |
Germany |
xx% |
Automated microbiology analyzers (MALDI Biotyper) |
|
Qiagen |
Germany |
xx% |
Sample prep kits, syndromic PCR panels (QIAstat-Dx) |
|
Bio-Rad Laboratories |
U.S. |
xx% |
Quality control solutions (BioBall), MRSA/CPO detection |
|
Bruker |
U.S. |
xx% |
MALDI-TOF mass spectrometry (MBT Smart) |
|
Luminex (DiaSorin) |
Italy |
xx% |
Multiplex PCR (VERIGENE system) |
|
Meridian Bioscience |
U.S. |
xx% |
Gastrointestinal pathogen tests (Illumigene) |
|
Hologic |
U.S. |
xx% |
Women’s health microbiology (Panther Fusion) |
|
Trivitron Healthcare |
India |
xx% |
Affordable culture media, neonatal sepsis kits |
|
HiMedia Laboratories |
India |
xx% |
Culture media, antibiotic discs for emerging markets |
|
SD Biosensor |
South Korea |
xx% |
Rapid POC tests (STANDARD M10 for TB) |
|
BioSynergy |
Malaysia |
xx% |
ASEAN-focused antimicrobial susceptibility test kits |
Recent Developments
- In May 2024, Abbott revolutionized point-of-care testing with its ID NOW Strep A 2.0, a 5-minute strep throat detection capability. Rapidly adopted by 5,003 U.S. clinics, it boosted the company's POC market share by 12.3% by showing a 30.6% reduction in unnecessary antibiotic prescriptions.
- In March 2024, bioMérieux introduced a breakthrough in syndromic PCR testing, BIOFIRE SPOTFIRE System, after gaining FDA clearance, for respiratory and bloodstream infections. It captured 20.5% of U.S. hospital lab sales within six months while dramatically reducing diagnostic turnaround times from days to just one hour.
- Report ID: 5065
- Published Date: Jul 29, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Microbiology Testing Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert




