Lassa Fever Treatment Market Outlook:
Lassa Fever Treatment Market size was over USD 657.01 million in 2025 and is projected to reach USD 1.53 billion by 2035, growing at around 8.8% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of lassa fever treatment is evaluated at USD 709.05 million.
The growth of the market can be attributed to the growing cases of fever across the world is projected to promote market growth. According to the data by the World Health Organization (WHO), 1 in every 5 Lassa fever infections result in severe infection in organs. Moreover, in 80% of cases, no symptoms are reported. Symptoms can vary from mild symptoms, such as nausea, and vomiting, to severe symptoms, such as deafness, seizure, and coma. As per another report by the WHO, deafness occurs in 25% of the cases of Lassa fever.
In addition, there has been an increasing incidence of Lassa fever, backed by a lack of sanitation and proper healthcare facilities. The major cause of Lassa fever is the consumption of food or water contaminated with rodent urine or faces. According to the data by UNICEF, 5 out of 10 people did not have access to safe sanitation services, in 2020.
Key Lassa Fever Treatment Market Insights Summary:
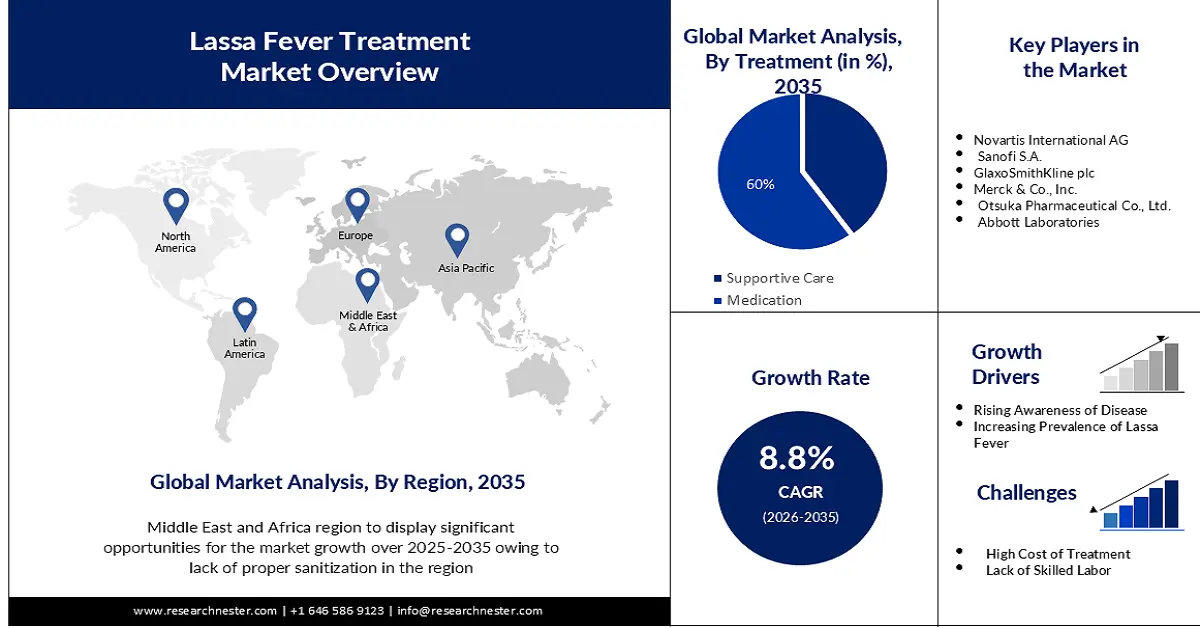
Regional Highlights:
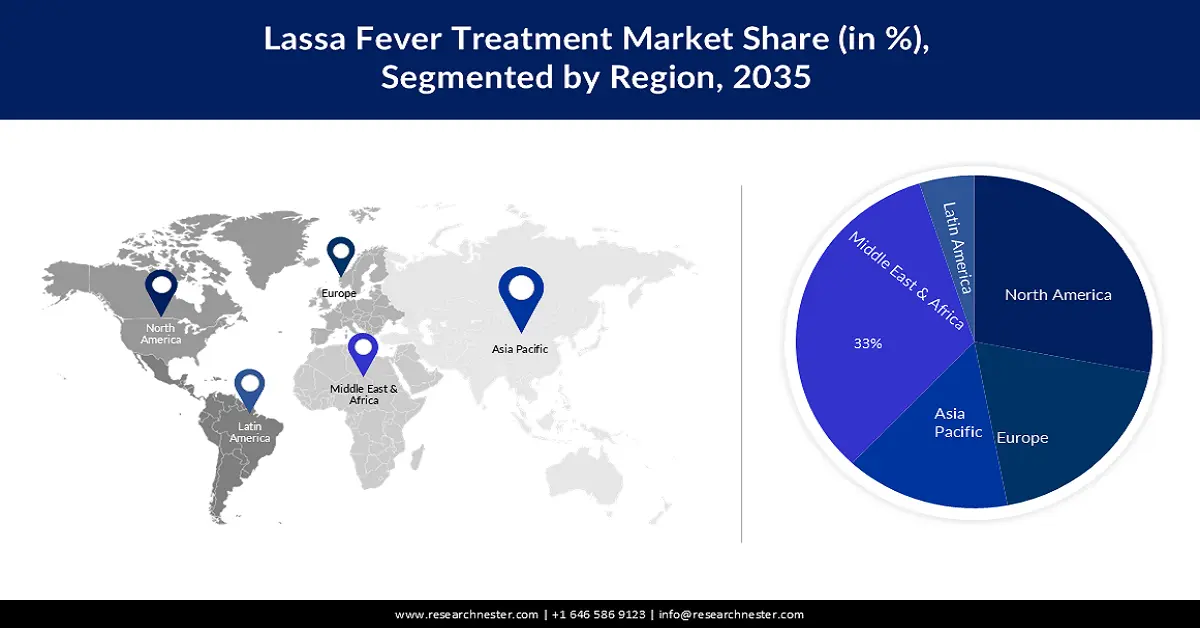
- The Middle East & Africa lassa fever treatment market will hold around 33% share, expected to grow by 2035, fueled by lack of sanitation services and rising healthcare investment.
- The North America market will register significant revenue share, set to expand by 2035, attributed to increasing R&D and supportive regulatory environment.
Segment Insights:
- The medication segment in the lassa fever treatment market is projected to achieve a 60% share by 2035, driven by the fast-acting relief and easy availability of medications.
- The hospital pharmacy segment in the lassa fever treatment market is projected to capture a 40% share by 2035, fueled by hospitals' access to candidate medicines and vaccines.
Key Growth Trends:
- Increasing Demand for Ribavirin
- Growing Awareness of Lassa Fever
Major Challenges:
- Increasing Demand for Ribavirin
- Growing Awareness of Lassa Fever
Key Players: Novartis International AG, Sanofi S.A., GlaxoSmithKline plc, Merck & Co., Inc., Otsuka Pharmaceutical Co., Ltd., Abbott Laboratories, Cipla Limited.
Global Lassa Fever Treatment Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 657.01 million
- 2026 Market Size: USD 709.05 million
- Projected Market Size: USD 1.53 billion by 2035
- Growth Forecasts: 8.8% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: Middle East & Africa (33% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, United Kingdom, France, Japan
- Emerging Countries: China, India, Brazil, Mexico, Indonesia
Last updated on : 9 September, 2025
Lassa Fever Treatment Market Growth Drivers and Challenges:
Growth Drivers
- Increasing Demand for Ribavirin - There has been surging demand for Ribarvirin tablets for Lassa fever treatment. Moreover, it is projected to be maintained in the forecast period, owing to ease of tablet production and handling, greater flexibility and compatibility, an increased amount of water solubility, and easier absorption by the body. Compared to the inhaler or oral solution, the manufacturing and handling of tablets is very easy.
- Growing Awareness of Lassa Fever - There is growing awareness of Lassa fever among healthcare professionals and the public. This awareness is leading to increased demand for diagnostic tests and treatment options.
- Increasing Investment Research and Development - The main drivers of the Lassa fever market over the forecast period are also multidisciplinary R&D activities to innovate new medicines and develop rapid immunodiagnostic kits, with a view to enhancing efficacy of existing treatment options for Lassa fever. There is a growing interest in developing new treatment for Lassa fever, and a number of clinical trials are currently underway. This investment in research and development is set to lead to the development of new and more effective treatments for Lassa fever in the future.
Challenges
- Limited Diagnostic Capabilities and Complexities with Clinical Trials - Accurate and timely diagnosis of Lassa fever is crucial for effective treatment. However, diagnostic capabilities in resource-limited endemic regions are often inadequate, leading to delayed diagnosis and treatment. This is predicted to hamper the market growth in the upcoming period. Conducting clinical trials for Lassa fever treatment also poses significant challenges due to the disease’s geographical distribution, limited infrastructure in affected areas, and ethical consideration regarding patient participation. This is also predicted to hamper the market expansion in the future times.
- High Cost of Treatment is predicted to Pose Limitations on the Market Expansion in the Future
- Lack of Specific Treatment is Set to Limit the Market Growth in the Forecast Period
Lassa Fever Treatment Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
8.8% |
|
Base Year Market Size (2025) |
USD 657.01 million |
|
Forecast Year Market Size (2035) |
USD 1.53 billion |
|
Regional Scope |
|
Lassa Fever Treatment Market Segmentation:
Treatment Segment Analysis
The medication segment is predicted to hold 60% share of the global lassa fever treatment market by 2035. The growth can be attributed on account of fast relief action of the drugs, quicker symptom elevation, and easy availability in pharmacies. Governments in endemic countries are taking steps to enhance the diagnosis and treatment of Lassa fever so that proper medication can be provided at the right time. This includes providing funding for research and development training healthcare workers and raising awareness of the disease.
Distribution Channel Segment Analysis
In terms of distribution channel, the hospital pharmacy segment in the lassa fever treatment market is estimated to reach a share of 40% during the forecast period. According to the World Health Organization, approximately 10% 15% of people infected with Lassa fever in Sierra Leone and Liberia are admitted into hospitals each year. For instance, pharmaceutical companies and scientists are constantly engaged in R&D activities such as the development of vaccines, close to patient diagnostic tests that have not yet been completed, candidate medicines being at an intermediate stage of testing, etc. These vaccines, and treatments will be easily available in the hospitals due to their financial capabilities.
Our in-depth analysis of the global market includes the following segments:
|
Symptoms |
|
|
Treatment |
|
|
Distribution Channel |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Lassa Fever Treatment Market Regional Analysis:
Middle East and African Market Insights
Middle East and Africa industry is poised to dominate majority revenue share of 33% by 2035. The lack of proper sanitation services in the region is also expected to significantly fuel the regional market growth. According to the statistics by the WHO, around 2 billion people do not have basic sanitation facilities, such as toilets, especially in middle and low-income economies. Improving healthcare facilities, backed by increasing investment in the sector is further estimated to boost the market growth. According to the data by the World Bank, USD 520.89 was spent per capita on healthcare in the Middle East and North Africa region, in 2018.
North American Market Insights
The lassa fever treatment market in the North America region is set to hold a significant revenue share by 2035. The growth of this market can attributed to the account of increasing presence of market players in this product in the North American region. Furthermore, Increasing research and development activities on the treatment of Lassa fever along with expansion of healthcare infrastructure and skilled professionals. The healthcare expenditure in North America is rising which is providing more resources for the development and advancement of Lassa fever treatment. Furthermore, the regulatory environment in North America is supportive of the development of new drugs which is encouraging pharmaceutical companies to invest in Lassa fever research. Besides, increasing demand for personalized medicine, is leading to the development of more targeted and effective treatments for Lassa fever in the North America region.
Lassa Fever Treatment Market Players:
- Pfizer, Inc.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Teva Pharmaceutical Industries Ltd.
- Mylan N.V.
- Novartis International AG
- Sanofi S.A.
- GlaxoSmithKline plc
- Merck & Co., Inc.
- Abbott Laboratories
- Inovio Pharmaceuticals Inc
- Cipla Limited
Recent Developments
- INOVIO, a biotechnology company focused on the development and commercialization of DNA medicines for the treatment and prevention of infectious diseases, cancer, and HPV-related diseases, today announced a collaboration with its partner Epidemic Preparedness Innovations Coalition to develop DNA medicines announced that they had signed an agreement to cancel the event. Following initial analysis of data from a study conducted by INOVIO and funded by CEPI, a product candidate for Lassa fever and Middle East respiratory syndrome has been announced.
- IAVI, a nonprofit scientific research organization, is conducting a Phase I clinical trial, called IAVI C102, using a novel Lassa fever virus vaccine with support from IAVI, in which volunteers from the PREVAIL clinical trial facility at Redemption Hospital in Monrovia, Liberia announced that they are participating in. Candidates from the Coalition for Infectious Disease Prevention Innovations received vaccinations. This study, sponsored by IAVI, is part of an existing partnership with CEPI that began in 2018 and is a global consortium of IAVI and partners to advance the development of IAVI's LASV vaccine candidate through clinical trial support Phases I and II. Provides up to USD 61.7 million to support.
- Report ID: 3641
- Published Date: Sep 09, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Lassa Fever Treatment Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




