Toxoplasmosis Treatment Market Outlook:
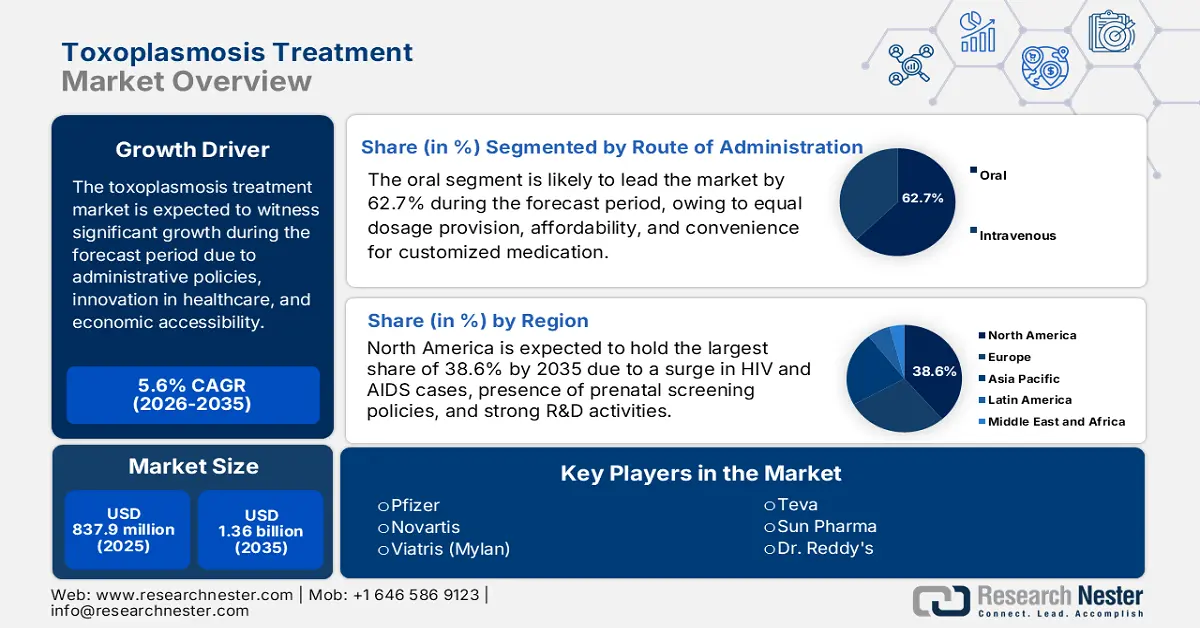
Toxoplasmosis Treatment Market size was valued at USD 837.9 million in 2025 and is projected to reach USD 1.36 billion by the end of 2035, rising at a CAGR of 5.6% during the forecast period, i.e., 2026-2035. In 2026, the industry size of toxoplasmosis treatment is expected to reach USD 884.8 million.
The toxoplasmosis treatment market’s growth is highly attributed to epidemiological and clinical drivers, followed by technological innovation in healthcare, regulatory policies, and economic accessibility. According to a report published by the World Health Organization (WHO) in April 2025, almost 260,000 women die during pregnancy and childbirth, and an estimated 92% of maternal deaths occur across low- and lower-middle-income nations as of 2023. For instance, in the same year, there were 82%, accounting for 225,000 maternal deaths, in South Asia and Sub-Saharan Africa. Meanwhile, Sub-Saharan Africa accounted for 70% of maternal deaths and South Asia constituted 17%, thereby suitable for the market’s demand globally.
Moreover, the aspect of advancements in diagnostics, particularly in healthcare, is also readily driving the market’s exposure. As per an article published by NLM in October 2022, the pulse oximeter utilization results in evaluating the oxygen saturation level, which is over 89%. Besides, machine learning is yet another innovation that assists doctors in conducting diagnoses and also caters to a challenge, wherein 5% of all patients account for 50% of overall expenses. Meanwhile, 80% of medical data is categorized in semi-structured and unstructured formats through patient data profiling, thus denoting a positive impact on the market internationally.
Key Toxoplasmosis Treatment Market Insights Summary:
Regional Highlights:
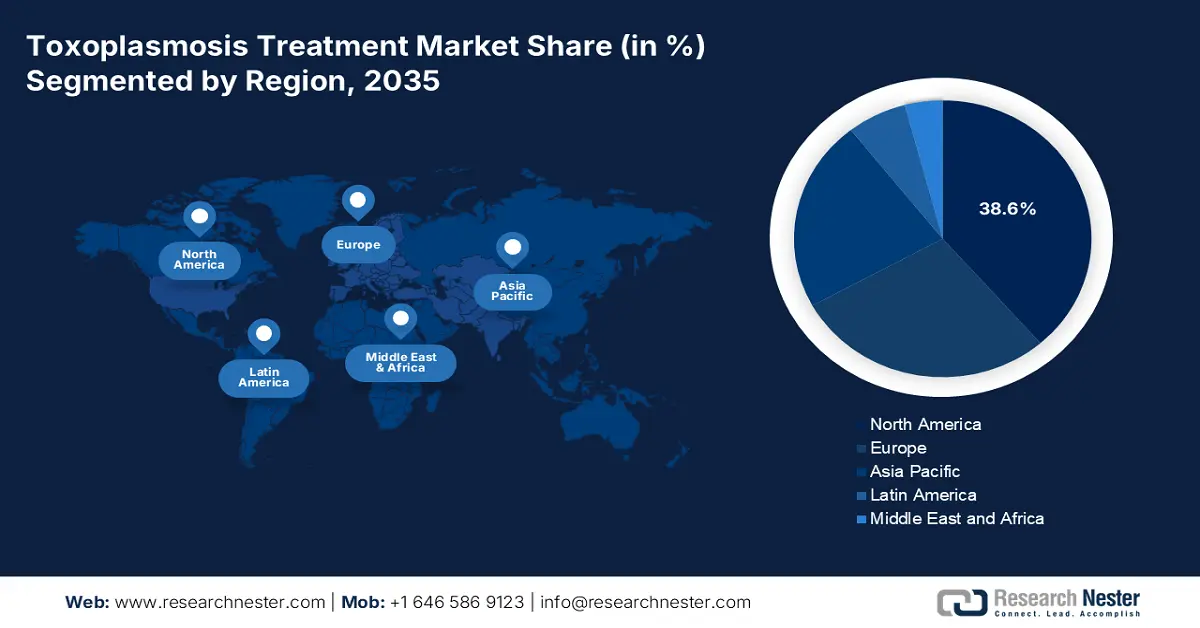
- North America in the Toxoplasmosis Treatment Market is projected to dominate with a 38.6% share by 2035, owing to rising HIV and AIDS prevalence, stringent prenatal screening regulations, and extensive R&D advancements in healthcare.
- Asia Pacific is estimated to secure a 22.4% share by 2035, impelled by increasing seroprevalence rates, a growing immunocompromised population, and harmonized drug approval processes.
Segment Insights:
- The oral segment in the Toxoplasmosis Treatment Market is anticipated to capture a 62.7% share by 2035, propelled by its equitable dosage delivery, cost-effectiveness, and convenience as the most prevalent route of medication administration.
- The hospitals and clinics segment is projected to account for a 58.2% share by 2035, supported by expanding healthcare accessibility and the integration of advanced technologies to manage chronic diseases.
Key Growth Trends:
- Advanced companion diagnostics commercialization
- Increase in telemedicine adoption
Major Challenges:
- Patient cost-effectiveness and increased out-of-pocket expenses
- Limitations in orphan drug designation
Key Players: Pfizer Inc. (U.S.), Novartis AG (Switzerland), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), Sun Pharmaceutical Industries Ltd. (India), Dr. Reddy's Laboratories Ltd. (India), Cipla Ltd. (India), Hetero Labs (India), Sanofi S.A. (France), Glenmark Pharmaceuticals Ltd. (India), Aurobindo Pharma Ltd. (India), Lupin Ltd. (India), Amneal Pharmaceuticals, Inc. (U.S.), Zydus Cadila (India), Endo International plc (Ireland).
Global Toxoplasmosis Treatment Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 837.9 million
- 2026 Market Size: USD 884.8 million
- Projected Market Size: USD 1.36 billion by 2035
- Growth Forecasts: 5.6% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (38.6% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, China, Japan, United Kingdom
- Emerging Countries: India, South Korea, Brazil, Australia, Mexico
Last updated on : 5 September, 2025
Toxoplasmosis Treatment Market - Growth Drivers and Challenges
Growth Drivers
- Advanced companion diagnostics commercialization: This is essential in the modern healthcare system to deliver customized medicines by successfully predicting patients’ responses to specific drugs, thereby suitable for the market’s exposure. As per an article published by the European Journal of Operational Research in May 2025, the companion diagnostics market is expected to increase to USD 10.0 billion by the end of 2026, owing to licensing and partnership, wherein pharmaceutical organizations tend to collaborate with diagnostic players to create new medical solutions, catering to the market’s flourishing.
- Increase in telemedicine adoption: This driver optimizes the accessibility to provide care, particularly for remote patients and those with mobility challenges, denoting a huge opportunity for the market’s growth. According to the April 2023 ASPE Government report, there has been an increase in telehealth services, which are covered by Medicaid by 28.3%, as well as 26.8% for Medicare services. Besides, within the past 4 years, the national telehealth utilization rate in the U.S. was 27% among adults more than 18 years of age. Therefore, with its increased demand, telemedicine has a huge scope for uplifting the overall market globally.
- Shift towards health and medical agreements: This shift assists in enhancing coordination in health services internationally, formalizing responsibilities, and ensuring smooth transition between facilities and patient care. For instance, in June 2025, Quantum Health effectively signed a standard agreement to acquire Embold Health. The purpose of this acquisition was to deepen Quantum’s funding for AI-based personalization, which was significant for optimizing its Real-Time Intercept (RTI), along with provider engagement abilities to provide affordable and quality care to patients.
Maternal Cases Uplifting the Toxoplasmosis Treatment Market
Region-Wise Infection Distribution and Maternal Deaths, 2024
|
Components |
Overall |
Africa |
America |
East Mediterranean |
Europe |
Southeast Asia |
West Pacific |
|
Countries |
43 |
13 |
10 |
6 |
5 |
5 |
4 |
|
Number of Hospitals |
408 |
126 |
88 |
46 |
57 |
35 |
56 |
|
Women with maternal infection |
2,466 |
718 |
788 |
327 |
316 |
195 |
122 |
|
Less severe infection |
1,512 |
426 |
511 |
205 |
179 |
129 |
62 |
|
Complicated infections |
577 |
151 |
192 |
74 |
87 |
45 |
38 |
|
Severe maternal infection |
377 |
141 |
95 |
48 |
50 |
21 |
22 |
|
Maternal death infection |
26 |
16 |
3 |
1 |
1 |
3 |
2 |
Source: NLM
Challenges
- Patient cost-effectiveness and increased out-of-pocket expenses: There is absence of patient accessibility guarantee even when a product achieves reimbursement, which has caused a hindrance in the market. Besides, deductible healthcare plans are increasingly common, which denotes that patients are needed to pay increased out-of-pocket before insurance coverage commences. Meanwhile, in the case of a specialized drug, co-insurance still amounts to increased expenses every month, which has developed affordability barriers, thus negatively impacting the overall market.
- Limitations in orphan drug designation: The existence of the Orphan Drug Act effectively provides years of market exclusivity as well as tax credits, along with fundamentally defining the target population. This designation, frequently caps the toxoplasmosis treatment market’s potentiality, which makes business care in risk conditions and less attractive to pharmaceuticals. In addition, the increased expenses of clinical trials is difficult to justify for predefined and small patient group, which in turn, is affecting the market’s development.
Toxoplasmosis Treatment Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
5.6% |
|
Base Year Market Size (2025) |
USD 837.9 million |
|
Forecast Year Market Size (2035) |
USD 1.36 billion |
|
Regional Scope |
|
Toxoplasmosis Treatment Market Segmentation:
Route of Administration Segment Analysis
The oral segment in the toxoplasmosis treatment market is anticipated to garner the largest share of 62.7% by the end of 2035. The segment’s growth is highly attributed to its ability to provide equitable dosage, along with its cost-effectiveness and convenience as the most commonly utilized medication administration route. According to an article published by the Journal of Controlled Release in December 2023, the present cutting-edge technology for a standard oral peptide delivery ensures 1% bioavailability when sourced as an oral tablet, thereby suitable for the overall segment’s growth.
End user Segment Analysis
The hospitals and clinics segment in the toxoplasmosis treatment market is projected to hold the second-largest share of 58.2% during the forecast period. The segment’s upliftment is effectively fueled by its importance in providing healthcare accessibility, ensuring routine check-ups, and managing chronic diseases by integrating advanced technologies. In this regard, the December 2024 CDC report stated that through stewardship, 85% of acute care hospitals comprise core elements to guide antibiotic effects in comparison to 41% over the past 7 years, thus denoting an increase in the segment’s demand across every nation.
Distribution Channel Segment Analysis
The hospital pharmacies segment in the toxoplasmosis treatment market is expected to account for the third-largest share of 50.2% by the end of the projected timeline. The segment’s development is propelled by the complicated and critical nature of diseases, which frequently require prenatal administration of advanced drugs, such as pyrimethamine and sulfadiazine. Besides, treatment management and initiation for severe episodes, especially among immunocompromised patients and congenital toxoplasmosis patients, have necessitated inpatient care for dosage monitoring and titration to combat side effects.
Our in-depth analysis of the global toxoplasmosis treatment market includes the following segments:
|
Segment |
Subsegment |
|
Route of Administration |
|
|
End user |
|
|
Distribution Channel |
|
|
Drug Class |
|
|
Indication |
|
|
Type |
|
|
Population |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Toxoplasmosis Treatment Market - Regional Analysis
North America Market Insights
North America in the market is projected to emerge as the most dominating region by accounting for the largest market share of 38.6% by the end of 2035. The market’s exposure in the region is highly attributed to an increase in HIV and AIDS prevalence, stringent prenatal screening reforms, robust research and development (R&D) activities in pharmacy, and innovative healthcare facilities. As per a report published by Genetics in Medicine in February 2023, noninvasive prenatal screening has been useful for detecting NT sonogram during first trimester within a range of 64% to 70%, followed by 82% to 87% for NT and serum analytes, and 69% for maternal serum AFP, thereby suitable for the market’s growth.
The market in the U.S. is steadily growing, owing to an expansion in Medicaid and Medicare services, FDA-approved drugs, an increase in the immunocompromised population, and private insurance reimbursement policies. Besides, the June 2023 NLM article reported that over 2.8 million antibiotic-resistant infections occur every year, and over 35,000 people die due to this condition. Besides, the August 2024 MACPRAC report denoted that Medicaid tends to lower unnecessary expenses, which range between USD 600 billion to more than USD 1.9 trillion every year, thereby managing them wisely in the country’s healthcare system.
The market in Canada is also growing due to the presence of provincial universal healthcare, neonatal screening reforms, biosimilar integration, and government investment. According to the March 2025 Government of Canada report, the Infectious Disease and Climate Change Fund made an investment of USD 520,248 to the Acadia University to assess the expansion in mosquito and vector potential range between April 2020 and March 2025. Likewise, the regulatory body funded USD 1,263,080 to Bishop’s University for ensuring citizen-based Ixodes scapularis surveillance between September 2018 to March 2024. Therefore, with such fund provision from the government, there is a huge opportunity for the market to flourish.
HIV Incidences in North America
|
U.S. |
Canada |
||
|
Components |
Numbers/Percentage |
Components |
Numbers/Percentage |
|
HIV Infection |
31,800 |
HIV Incidence |
1,833 |
|
Male-to-male contact |
67% |
National rate |
4.7 per 100,000 population |
|
Heterosexual contact |
22% |
Diagnoses in males |
6.3% for 1,224 new cases |
|
Injected drugs |
7% |
Diagnoses in females |
3.1% for 597 new cases |
|
New HIV cases by 2030 |
3,000 |
Children affected |
0.2 per 100,000 population |
Sources: CDC, April 2024; Government of Canada, December 2023
APAC Market Insights
Asia Pacific in the toxoplasmosis treatment market is projected to be the fastest-growing region by garnering a share of 22.4% by the end of the forecast duration. The market’s upliftment in the region is propelled by a surge in seroprevalence rates, a rise in the immunocompromised population, improved diagnostic capabilities, and harmonization of drug approvals. As per an article published by Science for Optimal Cancer Care in February 2024, 78.5% of distal and 62.1% of proximal gastric cancer are attributed to H. pylori infection. In addition, the H. pylori prevalence in distal GC cases in China was 66.5%, thereby denoting a huge demand for the market in the region.
The market in China is significantly growing, owing to the existence of the Health China 2030 initiative by the central government, massive regional pharmaceutical manufacturing, and an expansion in public health insurance. According to the April 2022 NLM article, the duration for including innovative drugs in the National Reimbursement Drug List (NRDL) has been reduced from 4 to 5 years to within the clearance year. Besides, the Center for Drug Evaluation (CDE) introduced 100 new drugs as of 2022 through R&D consultations and guidelines. Meanwhile, the proportion of domestic new drugs applying for their first IND was 79% in the same year, thus suitable for the market’s growth.
The market in India is also growing due to generic dominance, increase in cost sensitivity, fragmentation in the public health system, and pioneering role in the WHO’s prequalified generic manufacturing. As per the June 2025 PIB report, the Ayushman Bharat- Pradhan Mantri Jan Arogya Yojana (PM-JAY) scheme has created 41.0 crore (410 million) health cards, along with treatments worth ₹1.19 crore (USD 136,767), health coverage of ₹5 lakhs (USD 5,747) for per family every year, and the presence of 31,958 hospitals. Therefore, with such facilities and developments, the market is expected to gain increased exposure in the overall country.
Europe Market Insights
Europe in the toxoplasmosis treatment market is projected to account for a considerable share of 28.4% during the forecast timeline. The market’s development in the region is highly driven by the presence of a centralized EMA administrative pathway, health programme funding facilities, harmonized clinical policies, and increased cross-border healthcare mobility. According to the August 2025 European Commission report, the HaDEA successfully published the EU4Health call for tenders to effectively implement digital health strategies, with an indicated budget accounting for €1,000,000. This also includes operations related to the European Health Data Space (EHDS) Regulation, thereby catering to the market’s growth in the region.
The market in Germany is steadily growing, owing to the presence of the Federal Joint Committee (G-BA) and early benefit assessment (AMNOG), robust organ drug incentives, and decentralized hospital formulary decisions (G-DRG). As per the December 2024 NLM article, the rare disease prevalence in Europe Union is more than or equal to 5 per 10,000 population, due to which 63 orphan drugs have been approved. In addition, the German Act on the Reform of the Market for Medicinal Products was formed, and newly cleared orphan drugs underwent an early benefit assessment under the act.
The toxoplasmosis treatment market in France is also growing due to compulsory nationwide prenatal screening law, the presence of a transparency committee and ASMR rating system, along with standard research institutes. According to the May 2023 NLM article, almost 800,000 children are screened every year, including 32,500 in Brittany. Generally, neonatal screening takes place between 48 hours and 72 hours of life. Besides, French neonatal screening assists in detecting sickle cell disease, congenital adrenal hyperplasia, phenylketonuria, and cystic fibrosis, thereby suitable for the overall market’s exposure.
Measuring or Checking Equipment 2023 Export and Import Data
|
Countries |
Export |
Import |
|
Germany |
USD 4.5 billion |
USD 2.0 billion |
|
UK |
USD 1.0 billion |
USD 881 million |
|
France |
USD 646 million |
USD 697 million |
|
Switzerland |
USD 884 million |
USD 313 million |
|
Italy |
USD 824 million |
USD 749 million |
|
Netherlands |
USD 565 million |
USD 728 million |
|
Hungary |
USD 454 million |
USD 269 million |
Source: OEC, May 2025
Key Toxoplasmosis Treatment Market Players:
- Pfizer Inc. (U.S.)
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Novartis AG (Switzerland)
- Mylan N.V. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sun Pharmaceutical Industries Ltd. (India)
- Dr. Reddy's Laboratories Ltd. (India)
- Cipla Ltd. (India)
- Hetero Labs (India)
- Sanofi S.A. (France)
- Glenmark Pharmaceuticals Ltd. (India)
- Aurobindo Pharma Ltd. (India)
- Lupin Ltd. (India)
- Amneal Pharmaceuticals, Inc. (U.S.)
- Zydus Cadila (India)
- Endo International plc (Ireland)
The international market is effectively fragmented, which is characterized by established generics manufacturers dominance, and notable players focusing on high-value and niche segments. Besides, geographic extension into high-prevalence emerging economies, along with generous investments in novel drug delivery systems are a few notable strategies to enhance the safety profiling for existing antiparasitics. In addition, tactical collaborations with diagnostic players are essential for developing integrated screening-to-treatment pathways. Meanwhile, in Europe and the U.S., organizations are implementing Orphan Drug Designations for precise indications to achieve market exclusivity and favorable pricing, which is positively impacting the overall toxoplasmosis treatment market.
Here is a list of key players operating in the global market:
Recent Developments
- In September 2024, Everise notified that it has successfully signed a binding agreement to acquire the healthcare vertical of Continuum Global Solutions (CGS) to enhance its capabilities in pharmacy benefit management.
- In April 2024, Pfizer Inc. declared that the Europe-based Commission has effectively granted marketing authorization for EMBLAVEO, which suitable for aiding adult patients with complicated intra-abdominal infections (cIAI).
- Report ID: 8060
- Published Date: Sep 05, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Toxoplasmosis Treatment Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




