Nontuberculous Mycobacterium Treatment Market Outlook:
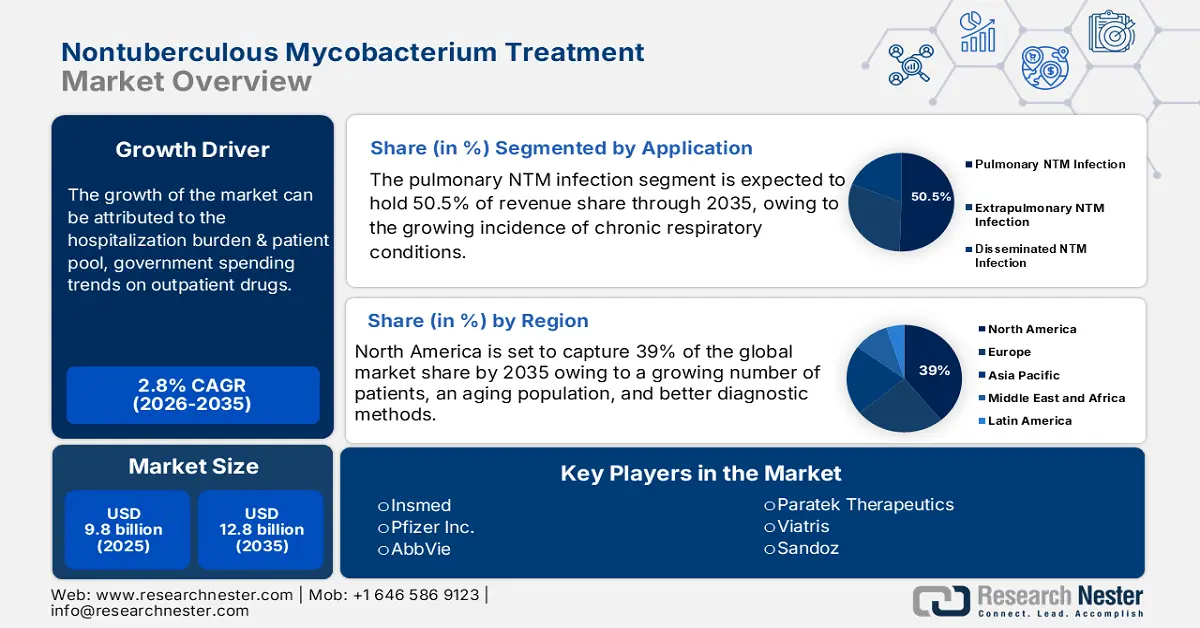
Nontuberculous Mycobacterium Treatment Market size was valued at USD 9.8 billion in 2025 and is projected to reach USD 12.8 billion by the end of 2035, rising at a CAGR of 2.8 % during the forecast period, i.e., 2026‑2035. In 2026, the industry size of nontuberculous mycobacterium treatment is evaluated at USD 10 billion.
The global nontuberculous mycobacterium treatment market is driven by an aging population and increased susceptibility in immunocompromised individuals. According to the American Lung Association report in October 2024, more than 86,000 people in the U.S. live with NTM lung disease, and this number is expected to rise more among women and the elderly population. This epidemiology directly informs the demand for complex, multi-drug regimens. The supply chain for NTM therapeutics is intricate, relying on a limited number of active pharmaceutical ingredient (API) producers for core antibiotics like macrolides (e.g., azithromycin) and aminoglycosides (e.g., amikacin).
On the trade side, the U.S. imports raw materials critical to antimicrobial manufacturing, such as specialty chemicals and intermediates, principally from Europe and Asia, and exports finished pharmaceuticals to markets with established regulatory schemes. Therapy for nontuberculous mycobacterium (NTM) pulmonary disease generally consists of extended antimicrobial treatment, and first-line treatments include a three-drug regimen of a macrolide (e.g., azithromycin or clarithromycin), rifampin or rifabutin, and ethambutol. According to the BC Center of Disease Control Report, the success rate with the drugs is the highest of 65.7%, which reflects the complexity of treatment and the requirement for new approaches. The increasing burden on the patient population and treatment makes the market demand.
Key Nontuberculous Mycobacterium Treatment Market Insights Summary:
Regional Highlights:
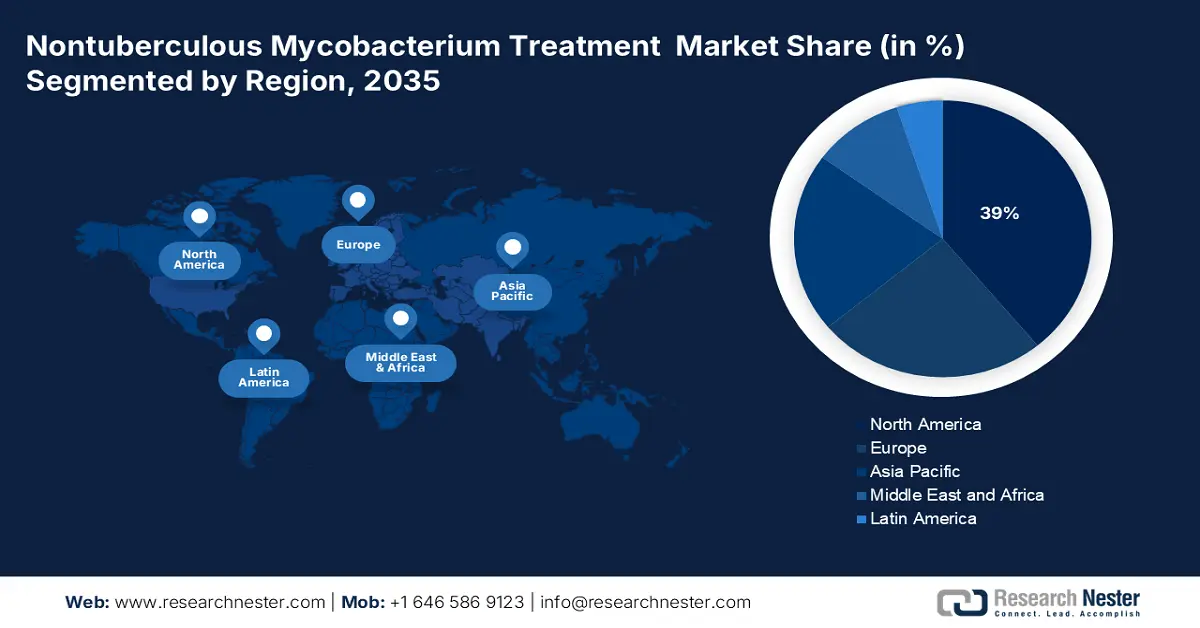
- North America is projected to hold the largest 39% share in the nontuberculous mycobacterium treatment market by 2035, stimulated by an increasing patient base, aging population, and advancements in diagnostic capabilities.
- Asia Pacific is expected to witness the fastest growth during 2026-2035, encouraged by expanding healthcare infrastructure, higher disease incidence, and greater government focus on infectious disease management.
Segment Insights:
- The pulmonary nontuberculous mycobacterium (NTM) infection segment is projected to account for 50.5% share by 2035 in the nontuberculous mycobacterium treatment market, propelled by the growing incidence of chronic respiratory conditions that heighten vulnerability to NTM infections.
- The hospitals segment is anticipated to capture a significant share by 2035, spurred by the increasing prevalence of specialized diagnostics and inpatient care requirements for NTM cases.
Key Growth Trends:
- Hospitalization burden & patient pool
- Government healthcare spending
Major Challenges:
Long treatment duration & adherence issues
Key Players: Insmed, Pfizer, AbbVie, Paratek Therapeutics, Viatris (Mylan/Upjohn), Sandoz (Novartis), Teva Pharmaceuticals, Cipla, Dr. Reddy’s Laboratories, Sun Pharmaceutical Industries, Fresenius Kabi, Hikma Pharmaceuticals, Sagent Pharmaceuticals, Amneal Pharmaceuticals, Baxter (Injectables Division), Shionogi, Takeda, Daiichi Sankyo, Otsuka Pharmaceutical, Astellas Pharma.
Global Nontuberculous Mycobacterium Treatment Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 9.8 billion
- 2026 Market Size: USD 10 billion
- Projected Market Size: USD 12.8 billion by 2035
- Growth Forecasts: 2.8% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (39% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Canada, Germany, United Kingdom, Japan
- Emerging Countries: China, India, South Korea, Australia, Brazil
Last updated on : 17 September, 2025
Nontuberculous Mycobacterium Treatment Market - Growth Drivers and Challenges
Growth Drivers
- Hospitalization burden & patient pool: The rising prevalence of NTM lung disease, especially among the elderly and immunocompromised, is a major demand driver. The World Bank Data in 2025 states that 10% of the global population is 65 years and above and the NIH report in November 2024 depicts that 14 million adults are having chronic pulmonary disease. This demographic shift sustains long-term market growth. This trend places additional strain on healthcare systems, highlighting the need for cost-effective and accessible NTM treatment options.
- Government healthcare spending: The overall budget request for fiscal year 2025 was roughly USD 9.683 billion, based on the March 2024 CDC budget report. The large investments were made to monitor and control infectious diseases, including respiratory pathogens like NTM. Presently, the CDC has increased the health expenditure in 2025 with USD 50 million on respiratory disease treatments. These expanded budget allocations and reimbursement policies underscore the public health emphasis on managing NTM disease through improved treatment access and innovation.
- Developments in diagnostic technology: Molecular testing, in particular, has greatly enhanced the early and precise diagnosis of nontuberculous mycobacteria (NTM) infections. Molecular methods such as real-time PCR and rpoB gene sequencing have demonstrated sensitivity and specificity rates exceeding 80%, respectively, compared to culture-based diagnostics, based on NLM May 2023 article. This increased accuracy facilitates prompt treatment initiation and improves patient outcomes, driving demand for targeted therapies.
Treatment Regimens and Clinical Considerations for NTM Pulmonary Disease
|
NTM Species |
Treatment Regimen for Associated Pulmonary Disease |
Comments |
|
Mycobacterium avium complex (MAC) |
Rifampicin + Ethambutol + Either clarithromycin or azithromycin |
• Non-severe disease: Three times per week regimen |
|
M. kansasii |
Rifampicin + Ethambutol + Either clarithromycin, azithromycin, or isoniazid (with pyridoxine) |
• Rifampicin-resistant M. kansasii: Three-drug regimen guided (but not dictated) by DST |
|
M. malmoense |
Rifampicin + Ethambutol + Either clarithromycin or azithromycin |
• Severe disease: Consider adding IV aminoglycoside |
|
M. xenopi |
Rifampicin + Ethambutol + Either clarithromycin or azithromycin + Either moxifloxacin or isoniazid |
• Severe disease: Consider adding IV aminoglycoside |
|
M. abscessus |
Initial phase: Amikacin (IV) + Tigecycline (IV) + Imipenem (IV) + Either clarithromycin or azithromycin |
• New drug to consider: omadacycline |
Source: NLM January 2024
Historical Patient Pool of NTM Disease
|
Species |
2017 |
2018 |
2019 |
2020 |
2021 |
2022 |
Total (%) |
|
M. intracellulare |
4 |
13 |
33 |
17 |
27 |
51 |
145 (24.7) |
|
M. avium |
0 |
9 |
29 |
31 |
32 |
53 |
154 (26.2) |
|
M. scrofulaceum |
0 |
0 |
1 |
2 |
2 |
10 |
15 (2.6) |
|
M. chelonae / M. abscessus |
5 |
11 |
29 |
23 |
31 |
52 |
151 (25.7) |
|
M. Kansasii |
1 |
1 |
8 |
5 |
10 |
12 |
37 (6.3) |
|
M. fortuitum |
0 |
0 |
3 |
4 |
5 |
7 |
19 (3.2) |
|
M. gordonae |
0 |
0 |
2 |
5 |
4 |
2 |
13 (2.2) |
|
M. lentiflavum |
0 |
0 |
0 |
3 |
3 |
4 |
10 (1.7) |
|
M. tuberculosis / NTM co-infection |
0 |
0 |
1 |
7 |
2 |
3 |
13 (2.2) |
|
Other species* |
1 |
3 |
4 |
7 |
6 |
9 |
30 (5.1) |
Source: NLM October 2023
Challenges
- Long treatment duration & adherence issues: High-cost treatment plans can put a strain on patients' access to care. Medicaid and Medicare often limit coverage to just a handful of medications or ask patients to chip in on costs, leaving many unable to afford the complete treatment. Many countries lack clear regulations for non-tuberculous mycobacterial (NTM) infections, which makes it even tougher for patients to find the support they need. The World Health Organization has emphasized how crucial it is to harmonize regulations for non-communicable disease (NCD) drugs to improve access for everyone.
Nontuberculous Mycobacterium Treatment Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
2.8% |
|
Base Year Market Size (2025) |
USD 9.8 billion |
|
Forecast Year Market Size (2035) |
USD 12.8 billion |
|
Regional Scope |
|
Nontuberculous Mycobacterium Treatment Market Segmentation:
Application Segment Analysis
The pulmonary nontuberculous mycobacterium (NTM) infection is dominating the segment and is expected to hold a share value of 50.5% by 2035. The growing incidence of chronic respiratory conditions that impair lung function and increase susceptibility to NTM infections is the main driver of this market. According to the August 2023 NLM report, the patients having chronic lung disease, and the annualized prevalence of pulmonary NTM infection is 6.1 cases per 100,000 people. Enhanced diagnostics and growing awareness have expanded the diagnosed patient base, supporting demand for targeted and combination therapies.
End user Segment Analysis
Hospitals dominate the end user segment and are likely to occupy a significant percentage by 2035. Hospitals play a key role in handling most cases owing to specialist diagnostics and care requirements. CDC infection surveillance (2025) points out that hospitals are the main treatment sites, indicating increased budgets allocated to infectious disease and pulmonary care. For example, the NLM report in March 2025 illustrates that culture conversion rates in hospital-treated NTM pulmonary disease patients range from 30% to 80%, depending on species and treatment adherence, indicating the complexity and significance of inpatient settings in fuelling market revenue.
Treatment Type Segment Analysis
Combination therapy, comprising macrolides, rifamycins, and ethambutol, is leading the treatment type segment. The segment is driven by its efficacy in decreasing antimicrobial resistance and enhancing clinical responses. The CDC and AHRQ are recommending such regimens as standard for pulmonary NTM management, highlighting their superiority to monotherapy. Increasing diagnosis rates, spurred by heightened awareness and increased testing, further drive this segment's growth. Treatment complexity demands multi-drug therapy to avoid resistance and relapse, supporting market growth and adoption.
Our in-depth analysis of the global nontuberculous mycobacterium treatment market includes the following segments:
|
Segments |
Subsegments |
|
Drug Type |
|
|
Treatment Type |
|
|
End user |
|
|
Distribution Channel |
|
|
Application |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Nontuberculous Mycobacterium Treatment Market - Regional Analysis
North America Market Insights
North America is anticipated to capture the highest share of 39% in the global nontuberculous mycobacterium treatment market by the end of 2035. The growth is fueled by a growing number of patients, an aging population, and better diagnostic methods. As per the report from Ontario in February 2024, Ontario increased investments of USD 110 million to benefit an additional 328,000 patients annually. Government initiatives are promoting early intervention and cost-effective treatment strategies, resulting in better patient outcomes and fewer hospitalizations. Further, rising public health awareness, enhanced diagnostic standards, and R&D for enhanced regimens also boost the market.
The nontuberculous mycobacterium treatment market in the U.S. is driven by an aging population experiencing chronic respiratory infection. Enhanced screening and diagnostic capabilities, supported by federal investment, rising detection rates of cases, and expanding treatment. The American Lung Association report of 2025 describes that 35.2 million individuals suffer from chronic lung disease in 2023, and the number of death cases enrolled through lung disease in 2022 was 586,000. This increasing disease burden emphasizes the urgent requirement for novel therapies and continued healthcare investment in the NTM treatment environment.
Prevalence of NTM disease in 2023
|
Country |
Prevalence |
|
U.S. |
3.1/100,000 to 4.7/100,000 |
|
Canada |
19.0 cases/100,000 persons |
Source: NLM September 2023, NLM July 2023,
APAC Market Insights
Asia Pacific is the fastest-growing region in the global nontuberculous mycobacterium treatment market throughout the discussed period. The expansion in this area is created by a combination of rising disease rates, the growth of healthcare infrastructure, and an increased emphasis by governments on infectious disease controls. Significant factors contributing to this growth include improvements in diagnosis, increased education of healthcare providers, and increased investment in drug development and access programs.
Over the last five years, the government in China has increased spending on NTM treatment. The national programs have built a pathway for access to treatment through healthcare changes and insurance policies. As more people live in cities and pollution and respiratory problems evolve into an epidemic, the number of NTM patients is expected to climb as well. As per the NLM report in May 2024 states that the prevalence of NTM cases in China was 6.4%. The large at-risk population due to COPD and aging contributes to significant market growth potential.
Europe Market Insights
The Europe nontuberculous mycobacterium treatment market is expected to display steady growth as a result of increased disease prevalence, an aging population. Healthcare systems across the region are increasing their budgets to meet the demands of the growing NTM treatment population. To meet this need, the European Commission is prioritizing health-based initiatives that focus on increasing NTM research and improving patient care pathways. There are other factors fueling market growth, including government reimbursement policies and an increasing knowledge base of clinicians regarding the complex treatment challenges associated with NTM infections. Current trends include personalized medicine and combination therapies to improve treatment outcomes.
Germany holds the largest share in the NTM treatment market within Europe in the forecast period. According to the NLM report in December 2023, the prevalence of NTM cases ranges between 5.3 to 5.8 per 100,000 population annually. The federal budget has expanded to include NTM-focused research and reimbursement programs, all of which help new drug therapies become accessible - positioning Germany as a growth center. The presence of many professional pharmaceutical companies focused on respiratory, infectious diseases, and combinations of both, is also an important facilitator for the market expansion.
Key Nontuberculous Mycobacterium Treatment Market Players:
- Insmed
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Pfizer
- AbbVie
- Paratek Therapeutics
- Viatris (Mylan/Upjohn)
- Sandoz (Novartis)
- Teva Pharmaceuticals
- Cipla
- Dr. Reddy’s Laboratories
- Sun Pharmaceutical Industries
- Fresenius Kabi
- Hikma Pharmaceuticals
- Sagent Pharmaceuticals
- Amneal Pharmaceuticals
- Baxter (Injectables Division)
- Shionogi
- Takeda
- Daiichi Sankyo
- Otsuka Pharmaceutical
- Astellas Pharma
The global marketplace in which nontuberculous mycobacterium operates under strong competitive forces. The industry leaders actively focus in developing new antibiotics and biologics through substantial research investments to address resistant NTM strains. Their business approach includes establishing biotech partnerships while acquiring new products and exploring Indian and Southeast Asian emerging markets. The market participants pursue partnerships with medical institutions and regulatory bodies to speed up drug approvals while expanding their international market presence.
Below is the list of some prominent players operating in the market:
Recent Developments
- In June 2025, BioVersys AG partnered with a Japan pharmaceutical company Shionogi & Co. to advance a groundbreaking preclinical ansamycin platform aimed at creating treatments for non-tuberculous mycobacteria (NTM). As part of this partnership, Shionogi will provide BioVersys with an upfront payment of USD 5 million for the development of BV500, BioVersys's candidate drug for NTM. Additionally, there are potential regulatory and sales milestones that could reach up to USD 479 million.
- In January 2025, MicuRx Pharmaceuticals received approval for MRX-5, an anti-infection drug for the treatment of non-tuberculous mycobacteria (NTM) infections. According to the news release, MRX-5 has a low risk of drug resistance and boasts high oral bioavailability. This makes it a strong candidate for long-term treatment of chronic infections.
- Report ID: 4072
- Published Date: Sep 17, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Nontuberculous Mycobacterium Treatment Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




