Neurointerventional Devices Market Outlook:
Neurointerventional Devices Market size was valued at USD 3.33 billion in 2025 and is likely to cross USD 5.37 billion by 2035, registering more than 4.9% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of neurointerventional devices is assessed at USD 3.48 billion.

The growth in the neurointerventional devices market is necessitated predominantly by the increased incidence of neurological disorders such as ischemic stroke and cerebral aneurysm. For instance, in April 2023, as per AHAIASA report, in 2019, 77.19 million people worldwide suffered an ischemic stroke, accounting for 3.29 million deaths and 63.48 million disability-adjusted life years. Moreover, diagnostic imaging advancements, particularly high-resolution angiography, make precise device guidance to maximize procedural success. For instance, in October 2024, using SCORE Opera angiography systems, Shimadzu Corporation created SMART Voice that increases the effectiveness of examinations and lessens the workload for medical professionals.
Moreover, demographic shifts such as increased geriatric patient populations increase the prevalence of cerebrovascular disease and therefore upsurge the denominator of patients. For instance, in October 2024, by 2050, 80% of older people will live in low- and middle-income countries. In addition, in 2020, there were more adults aged over 60 years than children under 5 years. Between 2015 and 2050, the proportion of the global population over 60 will nearly double, rising from 12% to 22%. Thus, the neurointerventional devices market is expected to grow steadily over the coming years owing to an aging population, ongoing research and development initiatives, and technical advancements.
Key Neurointerventional Devices Market Insights Summary:
Regional Highlights:
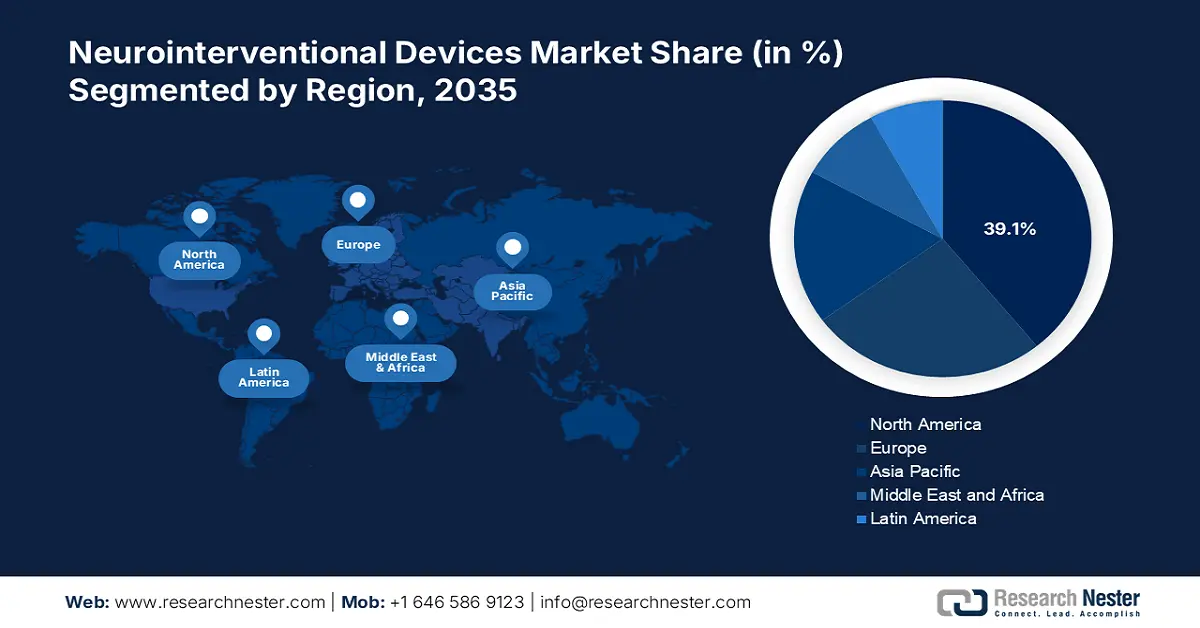
- North America holds a 39.1% share in the Neurointerventional Devices Market, driven by high prevalence of neurological disorders and advanced healthcare infrastructure, ensuring sustained growth through 2026–2035.
Segment Insights:
- The Stroke segment of the Neurointerventional Devices Market is projected to hold around 64.3% share by 2035, fueled by the acute presentation of ischemic stroke necessitating immediate treatment.
Key Growth Trends:
- Rising demand for minimally invasive procedures
- Surging R&D activities and funding
Major Challenges:
- Lack of skilled professionals
- Post-market surveillance
- Key Players: Johnson and Johnson Services Inc., Penumbra, Inc., Microport Scientific Corporation, Stryker, MicroVention Inc., Codman Neuro, and more.
Global Neurointerventional Devices Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 3.33 billion
- 2026 Market Size: USD 3.48 billion
- Projected Market Size: USD 5.37 billion by 2035
- Growth Forecasts: 4.9% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (39.1% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, China, Japan, Germany, United Kingdom
- Emerging Countries: China, India, Japan, South Korea, Singapore
Last updated on : 12 August, 2025
Neurointerventional Devices Market Growth Drivers and Challenges:
Growth Drivers
- Rising demand for minimally invasive procedures:One of the main growth catalysts in the neurointerventional devices market is the rising demand for minimally invasive procedures. Through such processes, patients recover from the treatment faster, experience fewer complications, and combat fewer scars. To enable efficient and targeted therapy, companies are encouraging innovations such as micro-stents and high-end catheters. For instance, in November 2022, iVascular increased the range of products it offers for the treatment of neurovascular diseases. These devices include iNdeep microcatheter, iNtercept retriever device, iNedit balloon distal access catheter, and iNstroke 4Fr and 6Fr aspiration catheter with CE-mark.
- Surging R&D activities and funding: The neurointerventional devices market is also witnessing a sharp growth graph being fueled by rising R&D activity and high investments. For instance, in April 2024, neuroClues announced that the latest €5 million (USD 5.2 million) funding round was successfully closed. Its innovative eye-tracking technology promised to help diagnose neurodegenerative diseases earlier. In addition, the emerging R&D boom focuses on improving the efficiency of devices, rising patient outcomes, and growing conditions treatable via the services of devices for neurological disorders. Thus, developments in biomaterials, neuroimaging, and robots are being integrated intensely.
Challenges
- Lack of skilled professionals:The most extreme challenge in the neurointerventional devices market is the perpetual lack of highly qualified professionals. Neurointerventional procedures demand very high levels of training and clinical expertise, thus serving as a limitation to service delivery. Highly qualified practitioners are hence scarce and beyond reach for high-level care, particularly in rural settings. The shortage also serves as a limitation to the proper application of new technology and procedures, thus inhibiting market penetration and terminating best patient outcomes. This deficit can be rectified through enhanced training programs and resource allocation to grow the market sustainably.
- Post-market surveillance: The inhibitor to the neurointerventional devices market is after-market surveillance. The design complexity of the devices and implantation into delicate neurological anatomy monitors long-term performance and safety. Poor systematic follow-up of patients and data collection undermine maximal surveillance capability. Hence, the detection of unforeseen but serious side effects may endanger patients. Moreover, anatomical variability and device delivery technique make real-world device performance interpretation difficult. Hence, post-marketing monitoring by improved registries, effective reporting systems, and cross-stakeholder data sharing ensure ongoing safety and effectiveness of neurointerventional devices.
Neurointerventional Devices Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
4.9% |
|
Base Year Market Size (2025) |
USD 3.33 billion |
|
Forecast Year Market Size (2035) |
USD 5.37 billion |
|
Regional Scope |
|
Neurointerventional Devices Market Segmentation:
Indication (Stroke, Brain Aneurysm)
Stroke segment is set to dominate neurointerventional devices market share of around 64.3% by the end of 2035. Acute presentation of ischemic stroke necessitates immediate re-establishment of cerebral perfusion, and fuels the demand for innovative thrombectomy devices and embolic protection devices. Therefore, next-generation clot retrieval devices and neuroimaging technologies are uncompromising needs to ensure maximal procedure efficacy and outcome. For instance, in April 2023, RapidAI announced that Rapid NCCT Stroke, a device to identify alleged intracranial hemorrhage (ICH) and bulky vessel occlusion (LVO) from value-based CT imaging, has received FDA 510(k) clearance. This is a significant addition to RapidAI's suite of non-contrast-based solutions for stroke and trauma care.
Application (Hospitals, Ambulatory Surgical Center (ASC)
Based on the application segment, the hospitals segment in neurointerventional devices market is anticipated to garner a lucrative share by 2035, attributable to the state-of-the-art infrastructure and large patient care facilities. It includes advanced imaging modalities, specialized operating rooms, and intensive care units to manage complicated neurointerventional procedures. Hospitals are therefore the point of origin for managing acute neurologic disorders such as ischemic stroke and intracranial hemorrhage with critical necessity. For instance, in the March 2024 WHO report, according to a significant study published in The Lancet Neurology, over 3 billion people globally lived with a neurological condition in 2021. Since 1990, there has been an 18% increase in the total number of neurological conditions, thus driving the demand of the market globally.
Our in-depth analysis of the global market includes the following segments:
|
Application |
|
|
Product |
|
|
Indication |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Neurointerventional Devices Market Regional Analysis:
North America Market Statistics
North America neurointerventional devices market is predicted to account for revenue share of around 39.1% by 2035. It is characterized by high growth, primarily spurred by the common prevalence of high vascular neurological disorders and the highly advanced healthcare infrastructure. Moreover, the rapid adoption of sophisticated thrombectomy devices in acute ischemic stroke due to a less regulated process, along with the widespread availability of stroke centers fuels the market demand.
The U.S. market is growing exponentially owing to the successful clinical trials by companies in incorporating innovative devices to bring efficacy in neurological disorders. For instance, in July 2024, Rapid Medical completed the first neurovascular cases in the USA following the FDA approval of an active access solution. For peripheral vascular and neuro procedures, the DRIVEWIRETM 24's deflectable tip provides excellent catheter and device navigation.
The Canada neurointerventional devices market is likely to witness substantial growth during the forecast period due to the supportive regulatory framework. For instance, in March 2023, the Minister of Health, Canada announced a USD 38.3 million investment over five years for a new Brain Health and Cognitive Impairment in Aging (BHCIA) Research Initiative through the Canadian Institutes of Health Research (CIHR) Institute of Aging. The initiative seeks to enhance the care and services provided to individuals with cognitive impairment by creating and launching strategic funding opportunities.
Asia Pacific Market Analysis
The Asia Pacific neurointerventional devices market is predominantly driven by the demand for affordable neurointerventional therapies. In addition, the growth is spurred by the development of the burgeoning middle-class population and enhanced access to specialist neurovascular care in urban areas of the region. Furthermore, the indigenous production of neurointerventional devices at a fast speed with a cost-reduction focus and accessibility of best-end care across the region is one of the major growth trends in the region.
The market in India is witnessing lucrative growth opportunities owing to the strategic fundings to foster innovative measures in the field. For instance, in December 2022, In-Med Prognostics announced to have raised USD 2.1 million led by Exxora. The funds were utilized to stabilize and diversify the product portfolio and access global markets. It utilized deep learning algorithms and machine learning to offer time-saving correct neuro analysis which helps in the assessment and early diagnosis of neurological diseases such as dementia, Alzheimer's, and Parkinson's at reasonable costs.
The neurointerventional devices market in China is experiencing fast-paced growth owing to the robust growth towards research and development, bolstering advancements and breakthroughs. For instance, in February 2025, China researchers found a new therapeutic target for Parkinson's disease and also identified a potentially promising small molecule drug. This discovery was made by researchers from Huashan Hospital under the Fudan University umbrella in Shanghai. Hence, the country is anticipated to attain phenomenal growth during the projected timeline.
Key Neurointerventional Devices Market Players:
- Medtronic
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Johnson and Johnson Services Inc
- Penumbra, Inc.
- Microport Scientific Corporation
- Stryker
- MicroVention Inc
- Codman Neuro
The neurointerventional devices market is characterized by a competitive ecosystem dominated by powerful multinationals and start-ups in the medical field. Collaborations with specialist companies strategically for quick build-out of product lines and access to new technology are perhaps the most prominent trends for growth. For instance, in May 2024, Brainomix recently revealed several new studies carried out in collaboration with academic stroke institutions. It confirmed the effectiveness of its Brainomix 360 system in raising stroke treatment rates for patients throughout networks.
Here's the list of some key players:
Recent Developments
- In March 2025, Genentech's TNKase thrombolytic received FDA approval for the treatment of acute ischemic stroke (AIS) in adults. TNKase is delivered as a single 5-second intravenous (IV) bolus, which is given as an IV bolus followed by a 60-minute infusion.
- In February 2025, Penumbra declared the release of the Access25 delivery microcatheter. It claims that Access25 is a single-lumen device that helps doctors reach the neurovasculature in order to administer Penumbra's 0.020-inch coil platform.
- Report ID: 7312
- Published Date: Aug 12, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Neurointerventional Devices Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




