Desmoid Tumor Treatment Market Outlook:
Desmoid Tumor Treatment Market size was valued at USD 2.66 billion in 2025 and is set to exceed USD 5.64 billion by 2035, expanding at over 7.8% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of desmoid tumor treatment is estimated at USD 2.85 billion.
The growth is mainly characterized by the increasing demand for advanced therapies powered by the cumulative occurrence of conditions including cancer, desmoid tumors, and other rare diseases. Furthermore, the advancements in novel therapeutics and technological developments in treatment procedures also uplift market growth.
Governments across the globe are furnishing regulatory support for optimal approvals and funding for several research programs. According to the NLM report in March 2024, desmoid tumor, which is a rare disease, accounts for 2 to 4 cases per million people, and accounts for 0.03% of all neoplasms. It further reported that the condition is affecting individuals aged 15 to 60 years women being highly affected than men. Additionally, 5% to 10% of these cases are associated with FAP (familial adenomatous polyposis). Hence, the study underscores the necessity of desmoid tumor treatment to surpass the rising instances.
Key Desmoid Tumor Treatment Market Insights Summary:
Regional Highlights:
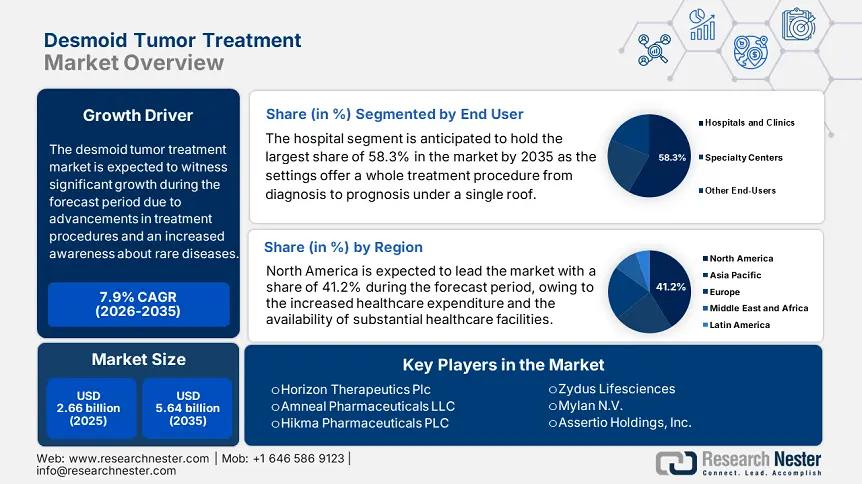
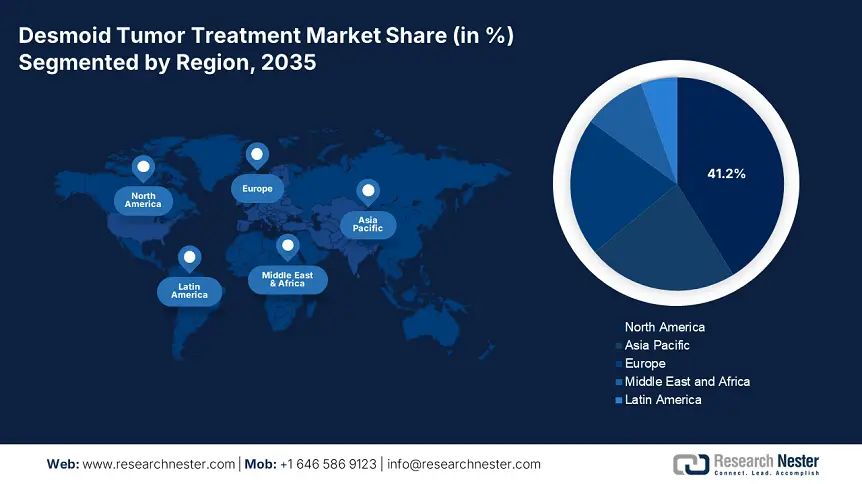
- North America dominates the Desmoid Tumor Treatment Market with a 41.2% share, driven by domestic investments in highly effective drugs and substantial healthcare facilities, fostering growth through 2026–2035.
- Asia Pacific’s desmoid tumor treatment market is expected to see substantial growth by 2035, driven by large patient population with rare diseases and demand for chemotherapy drugs.
Segment Insights:
- The Hospital segment is expected to capture 58.3% market share by 2035, fueled by the key role of hospital settings in providing comprehensive treatment processes.
- The Surgery segment is expected to grow at a considerable rate from 2026 to 2035, driven by the increased frequency of tumor removal through surgical procedures.
Key Growth Trends:
- Increasing awareness of diagnosis of rare diseases
- Advancements in targeted therapies
Major Challenges:
- Limited availability
- Elevated treatment expenses
- Key Players: Amneal Pharmaceuticals LLC, Hikma Pharmaceuticals PLC, Zydus Lifesciences, Mylan N.V.
Global Desmoid Tumor Treatment Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 2.66 billion
- 2026 Market Size: USD 2.85 billion
- Projected Market Size: USD 5.64 billion by 2035
- Growth Forecasts: 7.8% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (41.2% Share by 2035)
- Fastest Growing Region: North America
- Dominating Countries: United States, Germany, United Kingdom, France, Japan
- Emerging Countries: China, India, Brazil, Mexico, Russia
Last updated on : 12 August, 2025
Desmoid Tumor Treatment Market Growth Drivers and Challenges:
Growth Drivers
- Increasing awareness of diagnosis of rare diseases: The rising number of rare disease cases is creating a heightened awareness among patients and healthcare providers, driving the desmoid tumor treatment market. This boosts the demand for targeted treatments that can address the root cause of these rare diseases with appropriate treatment procedures. In February 2025, SpringWorks Therapeutics, Inc. announced a partnership with Jennifer Fisher, a desmoid tumor patient advocate, for an educational campaign. The launch aims to spread awareness about desmoid tumors and encourage early diagnosis and treatment, driving the demand for specialized therapeutic procedures.
- Advancements in targeted therapies: These advancements allow the firms to leverage the specialized expertise of healthcare providers, avoiding the long-term complications. Through these innovations, people can emphasize more effective and less invasive treatment options, and improve patient outcomes with enhanced clinical trials. For instance, in April 2021, Iterion Therapeutics, Inc., announced the successful completion of a Phase 1/2a clinical trial for Tegavivint, a nuclear beta-catenin inhibitor for desmoid tumor patients. It stated that the study confirmed the safety and tolerability, further augmenting the desmoid tumor treatment market growth.
Challenges
- Limited availability: One of the major challenges in the desmoid tumor treatment market is the limited availability of treatment procedures. This low availability makes it difficult to create standardized treatment protocols. Additionally, due to the complex nature of the disease, the clinical trials and regulatory processes are lengthy, restricting enhanced patient access and posing a significant challenge. As a result, the healthcare providers struggle to determine an effective treatment approach, which in turn hinders market expansion.
- Elevated treatment expenses: These complications occur due to the processes requiring highly specialized facilities and equipment, making the initial setup expenses substantial. The high costs associated with the advanced therapeutic process pose a major barrier in the desmoid tumor market, placing the burden on patients and healthcare providers. This makes it less accessible to those in need, creating healthcare disparities in the population and limiting the market growth further.
Desmoid Tumor Treatment Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
7.8% |
|
Base Year Market Size (2025) |
USD 2.66 billion |
|
Forecast Year Market Size (2035) |
USD 5.64 billion |
|
Regional Scope |
|
Desmoid Tumor Treatment Market Segmentation:
End User (Hospitals and Clinics, Specialty Centers)
Based on end user, the hospital segment dominated the desmoid tumor treatment market with the highest share of 58.3% by the end of 2035. The dominance is attributable to the key role of hospital settings in providing the whole treatment procedure from diagnosis to prognosis under one roof. Moreover, the technological advancements in the surgical procedures inspire the key players to launch more of such devices, uplifting the market growth. In April 2024, Asensus Surgical, Inc. entered into a definitive agreement with Sendai Tokushukai Hospital in Japan to tenure and utilize the Senhance Surgical System. The collaboration intends to advance surgical systems in the hospital settings for a greater outcome, widening the segment’s scope.
Treatment (Surgery, Radiation Therapy, Hormone Therapy)
Based on treatment, the surgery segment is projected to expand at a considerable rate during the forecast period in the desmoid tumor treatment market. The dominance is attributable to the increased frequency of tumor removal through the surgical procedures to improve quality of life. As per a 2022 Cancer Treatment and Research Communications report, surgery remains a viable and important treatment for desmoid tumors, particularly in symptomatic patients when tumors are located in critical areas such as the mesentery. It further stated that surgery can help achieve local control and preserve function where morbidity is limited. Thus, due to this companies are undertaking several initiatives to enhance their presence in the market.
Our in-depth analysis of the global desmoid tumor treatment market includes the following segments:
|
End User |
|
|
Diagnosis |
|
|
Treatment |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Desmoid Tumor Treatment Market Regional Analysis:
North America Market Analysis
North America is a key player in the desmoid tumor treatment market, projected to register a significant share of 41.2% during the forecast period. Domestic players in the region have amplified the market expansion with their increased investments in highly effective drugs and substantial healthcare facilities. In February 2024, SpringWorks Therapeutics, Inc., notified that EMA analyzed the Marketing Authorization Application for nirogacestat, an oral gamma-secretase inhibitor, for the treatment of adults with desmoid tumors based on Phase 3 DeFi trial in which it demonstrated significant results. Hence, such factors are anticipated to uplift the market significantly during the forecast period.
The desmoid tumor treatment market in the U.S. is growing exponentially due to the presence of several key players and their substantial research activities. The market is immensely supported by the governing bodies by maintaining an affordable price of the therapeutics and public-private collaborations. For instance, in March 2023, Thermo Fisher Scientific Inc., in partnership with the University of California, San Francisco, announced plans to accelerate the innovative cell-based therapies to treat various conditions such as rare diseases, cancer, and other disorders. Through these instances, the U.S. stands as a global hub for desmoid tumor treatment to advance disease management.
The Canada desmoid tumor treatment market is growing steadily, supported by the government due to an increased demand for advanced treatment procedures for rare diseases such as desmoid tumors. With a huge focus on positive patient outcomes, the collaborative team models are working to bring innovations in the field. In March 2025, the Government of Canada declared that Canada’s Minister of Health entered into an agreement with Quebec with an investment of USD 305 million for access to new and existing therapeutics, detection, and screening of rare diseases. Thus, with such support from the government, the country is anticipated to witness lucrative growth opportunities, accelerating market growth.
APAC Market Statistics
The Asia Pacific region in desmoid tumor treatment market accommodates a substantial number of patient populations diagnosed with several sorts of rare diseases, including desmoid tumors. This factor grants a lucrative market prospect for healthcare facilities providing treatment procedures, further driving market growth. Moreover, the region has a huge demand for chemotherapy drugs and targeted therapies due to the increased instances of cancer. Thus, these factors are boosting the region’s market.
The India desmoid tumor treatment market is mainly reinforced by a strong biotech sector. Supervisory modifications and monetary sustenance from the government have spurred the establishment of cutting-edge healthcare facilities, aiming to position India as a regional hub for desmoid tumor treatment. For instance, in August 2024, the Government of India reported that the Ministry of Health & Family Welfare initiated the National Policy for Rare Diseases, under which financial support of Rs. 50 Lakhs (USD 58,558.7) will be provided at 12 particular centers. It further stated that 1,118 patients have benefited from this policy, which significantly impacts the market growth.
The China desmoid tumor treatment market is driven by a large patient base with unmet medical needs. The country’s support for pharmaceutical innovation, combined with favorable regulatory approvals for faster clearance, has fostered an environment where biotech companies can rapidly advance the treatment. In February 2021, Apollomics, Inc. and Iterion Therapeutics announced their partnership to develop and commercialize Tegavivint, which is a selective inhibitor of nuclear β-catenin, in Mainland China, Hong Kong, Macau, and Taiwan. It is in Phase 1/2a clinical trial for desmoid tumor treatment. Hence, the country’s market is anticipated to witness considerable growth during the forecast period with such launch activities.
Key Desmoid Tumor Treatment Market Players:
- Horizon Therapeutics Plc
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Amneal Pharmaceuticals LLC
- Hikma Pharmaceuticals PLC
- Zydus Lifesciences
- Mylan N.V.
- Assertio Holdings, Inc.
- Ostro Health
- Iterion Therapeutics, Inc
- Apollomics, Inc.
- Alembic Pharmaceuticals Limited
- Apotex Inc.
- Bayer AG
- Thermo Fisher Scientific Inc.
- SpringWorks Therapeutics, Inc.,
- Sun Pharmaceutical Industries Ltd
- Baird Medical Investment Holdings Ltd.
- Immunome, Inc.
One of the key strategies adopted by companies involved in the desmoid tumor treatment market is investing in acquisitions to enhance their product portfolio. This is mainly done through acquiring the product from the key players and upgrading it to a higher efficacy to improve positive outcomes. For instance, in March 2024, Immunome, Inc. attained phase 3 asset AL102 and related drug candidate AL101 from Ayala Pharmaceuticals, Inc., which is a small-molecule gamma secretase inhibitor to enhance the treatment of desmoid tumors. These acquisitions, coupled with other strategic activities to influence positive significant market activities, maintain healthy competition between the players.
Below is the list of the prominent players in the desmoid tumor treatment market:
Recent Developments
- In March 2025, Baird Medical Investment Holdings Ltd. was awarded with Most Valuable Investment Award at the China Medical Industry Innovation Competition for the AI Tumor Ablation Surgical Robot.
- In January 2025, Ostro Health declared a partnership with Desmoid Tumor Research Foundation, aiming to increase awareness about desmoid tumors by bringing pro bono content to the DTRF website for critical information for patients and healthcare professionals.
- Report ID: 7597
- Published Date: Aug 12, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Desmoid Tumor Treatment Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




