AI-based Clinical Trials Solution Providers Market Outlook:
AI-based Clinical Trials Solution Providers Market size was valued at USD 2.73 billion in 2025 and is expected to reach USD 18.98 billion by 2035, expanding at around 21.4% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of AI-based clinical trials solution providers is evaluated at USD 3.26 billion.
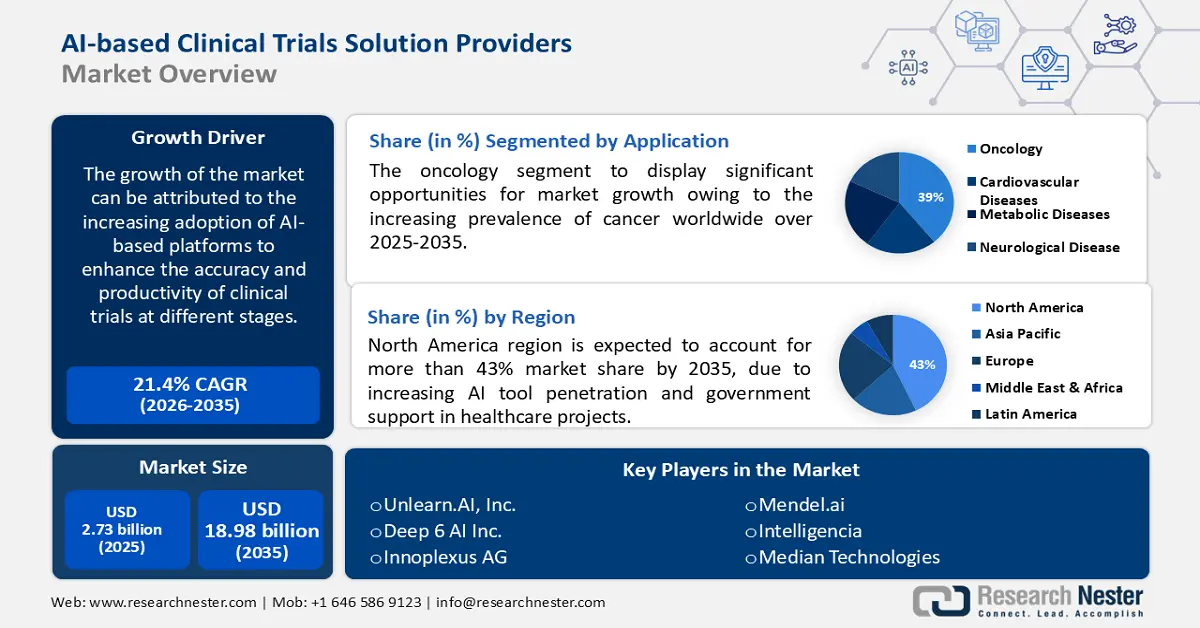
The growth of the market can be attributed to the increasing adoption of AI-based platforms to enhance the accuracy and productivity of clinical trials at different stages. According to the World Health Organization (WHO), the number of newly recruited trials registered on international clinical trial registry platforms (ICTRP) has been steadily increasing.
As the prevalence of various diseases increases, there is a need for new drug discoveries, which goes from a series of trials known as clinical trials, which tests potential treatments in human volunteers to see whether they should be approved for broader use in the general population. A treatment could be a drug, medical device, or biologic, such as a vaccine, blood product, or gene therapy. Potential treatments, however, must be studied in laboratory animals first to determine potential toxicity before they can be tried on people. As technology is advancing, AI-based clinical trials are becoming popular, where artificial intelligence and big data technology are used for extracting medical information. The market is growing owing to the prevalence of fetal diseases like cancer. According to studies, patient recruitment delays account for 85% of drug trial delays and 30% of early trial terminations. Patient eligibility and enrolment are two critical elements for the success of the overall drug study.
Key AI-based Clinical Trials Solution Providers Market Insights Summary:
Regional Highlights:
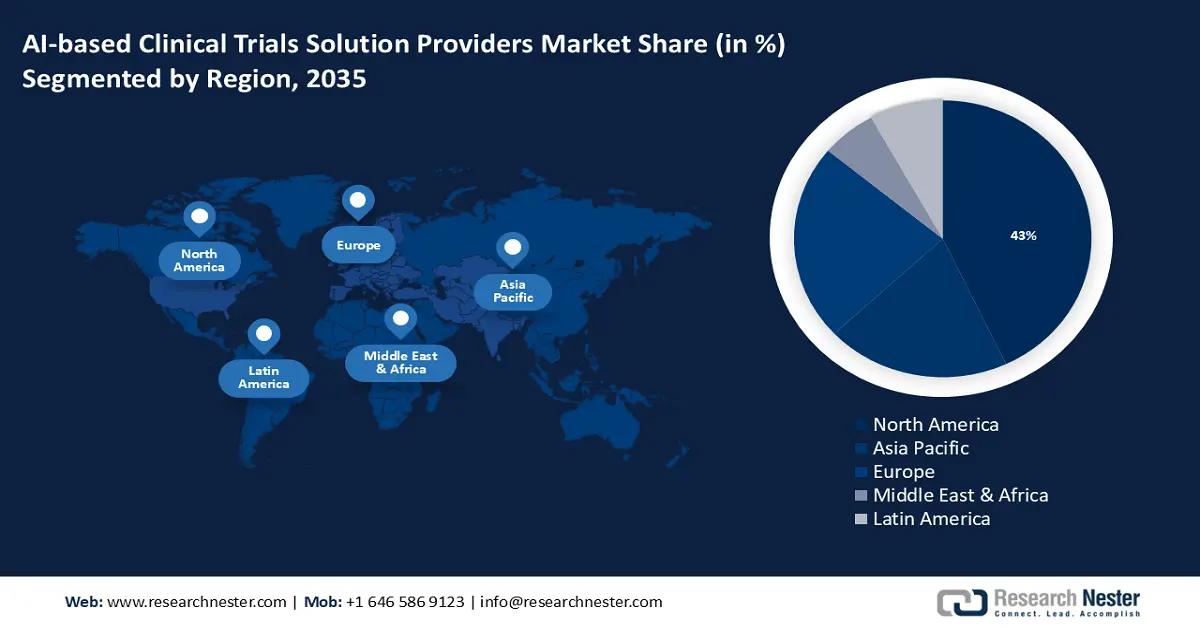
- North America AI-based clinical trials solution providers market will hold around 43% share by 2035, driven by increasing AI tool penetration and government support in healthcare projects.
- Asia Pacific market will capture significant revenue share by 2035, attributed to rising AI-based startups and supportive healthcare AI initiatives.
Segment Insights:
- The phase ii segment in the ai-based clinical trials solution providers market is forecasted to hold a 46.10% share by 2035, driven by the large number of clinical trials and the incorporation of AI tools for immediate results in drug trials.
- The oncology segment in the ai-based clinical trials solution providers market will experience substantial growth during 2026-2035, driven by the increasing prevalence of cancer and the growing number of drug trials utilizing AI-based equipment.
Key Growth Trends:
- Increasing Adoption of AI-based Platforms to Enhance Accuracy and Productivity at Different Stages - There is an increase in AI-based clinical trials for accuracy and productivity. Clinical trials are carefully designed, reviewed, and completed and need to be approved before they can start, which in turn requires higher accuracy, which AI-based platforms can easily do.According to the World Health Organization, the United States of America had the highest total number of trials registered during 1999
- Increasing Initiatives by Public and PrivateSectors
Major Challenges:
- Strict Regulations Across the Globe
- Privacy Related Concerns
Key Players: Unlearn.AI, Inc., Saama Technologies, LLC., Antidote Technologies, Inc., Biosymetrics, Pharmaceutical Pipeline Enhancement Strategies, LLC, Deep 6 AI Inc., Innoplexus AG, Mendel.ai, Intelligencia, Median Technologies.
Global AI-based Clinical Trials Solution Providers Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 2.73 billion
- 2026 Market Size: USD 3.26 billion
- Projected Market Size: USD 18.98 billion by 2035
- Growth Forecasts: 21.4% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (43% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, China, Germany, United Kingdom, Japan
- Emerging Countries: China, Japan, India, South Korea, Singapore
Last updated on : 9 September, 2025
AI-based Clinical Trials Solution Providers Market Growth Drivers and Challenges:
Growth Drivers
- Increasing Adoption of AI-based Platforms to Enhance Accuracy and Productivity at Different Stages - There is an increase in AI-based clinical trials for accuracy and productivity. Clinical trials are carefully designed, reviewed, and completed and need to be approved before they can start, which in turn requires higher accuracy, which AI-based platforms can easily do. According to the World Health Organization, the United States of America had the highest total number of trials registered during 1999–2021 (157,618), followed by China (80,333) and Japan (57,754). According to the same article, of trials categorized into a disease or condition, 81% were for non-communicable diseases, 15% for communicable, maternal, perinatal, and nutritional conditions, and 4% for injuries.
- Increasing Prevalence of Cancer Globally - There is an increase in the number of cancer cases globally, directly increasing the need for new drug development and treatment procedures for cancer. The higher drug development procedures increase clinical trials across the globe. According to the WHO, each year, approximately 400, 000 children develop cancer. The most common cancer varies among countries. Cervical cancer is the most common in 23 countries. Also, cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or almost one in six deaths.
- Increasing Initiatives by Public and Private Sectors – Globally, numerous governments are driving initiatives to enhance the region's clinical trial environment. The Australian Government, for instance, is promoting initiatives to enhance the clinical trial environment in Australia, enhance health results, and boost foreign investment in the nation. The Clinical Trials Project Reference Group (CTPRG), formerly known as the Clinical Trials Jurisdictional Working Group (CTJWG), brings together senior officials from Commonwealth, State, and Territory health departments, as well as the National Health and Medical Research Council (NHMRC). In order to identify and implement actions and system redesigns that will enable a streamlined and consistent national approach to clinical trials in Australia, to improve health outcomes and enhance productivity.
- Presence of a Large Number of Registered Clinical Trials – Prevalence of large number of registered clinical trials is expected to drive the growth of this market in the forecast period. According to the World Health Organization, 59,964 clinical trials were conducted worldwide in 2021.
Challenges
- Strict Regulations Across the Globe – These solutions come with multiple strict codes of conduct that need to be followed and they are not the same in all regions. The strict regulations associated with them is considered to hamper the growth of the market in the upcoming times.
- Privacy Related Concerns
- Lack of AI Implementation Framework
AI-based Clinical Trials Solution Providers Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
21.4% |
|
Base Year Market Size (2025) |
USD 2.73 billion |
|
Forecast Year Market Size (2035) |
USD 18.98 billion |
|
Regional Scope |
|
AI-based Clinical Trials Solution Providers Market Segmentation:
Application Segment Analysis
The oncology segment is anticipated to garner the largest market share over the forecast period, owing to the increasing prevalence of cancer worldwide and the increasing number of drug trials. In 2019, oncology accounted for the most popular therapy area for non-industry-sponsored clinical trials, estimated for 26% of all clinical trials. In the recent times, there has been rise in drug based clinical trials in oncological research, this in turn have raised the demand for AI-based equipment in the clinical research. Moreover, growing number of market players opting for oncology-based simulated instruments for clinical preliminaries is driving the growth.
Clinical Trial Phase Segment Analysis
The Phase II segment is expected to account for the fastest market share in the forecast period. Phase II held a market share of 46.1% in 2022, owing to the prevalence of a large number of clinical trials. The growing incorporation of AI tools for immediate results from the desired outcome of drug trials and also the collection of data at the same timeis driving growth of the segment. Additionally, Phase I segment is anticipated to show the fastest growth rate in the forecast period. As phase I is advantageous for patient retention, better trial design and patient recruitment, the ai-based solution is expected to grow in the forecast period.
Our in-depth analysis of the global market includes the following segments:
|
By Application |
|
|
By Clinical Trial Phase |
|
|
By End User |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
AI-based Clinical Trials Solution Providers Market Regional Analysis:
North American Market Insights
North America region is expected to account for more than 43% market share by 2035, owing to increasing penetration of AI-based tools and increasing government initiatives for adoption in different medical projects in the region. The region also has a rising corporate investment in artificial intelligence (AI) contributing market growth in the coming years. For instance, between 2018 and 2020, government agencies in the United States spent USD 1.9 billion on AI-related service obligations. Also, many ai-based startups opening their market in this region. For instance, BullFrog AI is a U.S. based ai startup, that creates bfLEAR an ai platform that allows precision medicines.
APAC Market Insights
Asia Pacific is also anticipated to hold the significant revenue shareby the end of 2035, owing to the increasing number of AI-based startups. The region also has an increasing awareness of AI-based clinical trials. Owing to the growing demand for AI tools and supportive government programs that focuses on the implementation of AI in the healthcare sector.
Europe Market Insights
Europe is anticipated to experience significant AI-based clinical trials solution providers market growth owing to the region's growing geriatric and target populations, expansion of key players' geographic markets, and active participation from governmental and nonprofit organizations.
AI-based Clinical Trials Solution Providers Market Players:
- Unlearn.AI, Inc.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Saama Technologies, LLC.
- Antidote Technologies, Inc.
- Biosymetrics
- Pharmaceutical Pipeline Enhancement Strategies, LLC
- Deep 6 AI Inc.
- Innoplexus AG
- Mendel.ai
- Intelligencia
- Median Technologies
Recent Developments
- BioAge to start the phase 2 trial of BGE-175, which can reverse the effect of immunity aging amongst elderly population, to treat COVID-19.
- AiCure to join hands with OncoBay Clinical, a contract research organization, to provide oncology sponsors with scalable, AI-powered insights to improve patient care and optimize drug development.
- Report ID: 3859
- Published Date: Sep 09, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
AI-based Clinical Trials Solution Providers Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




