Vaccine Adjuvants Market Outlook:
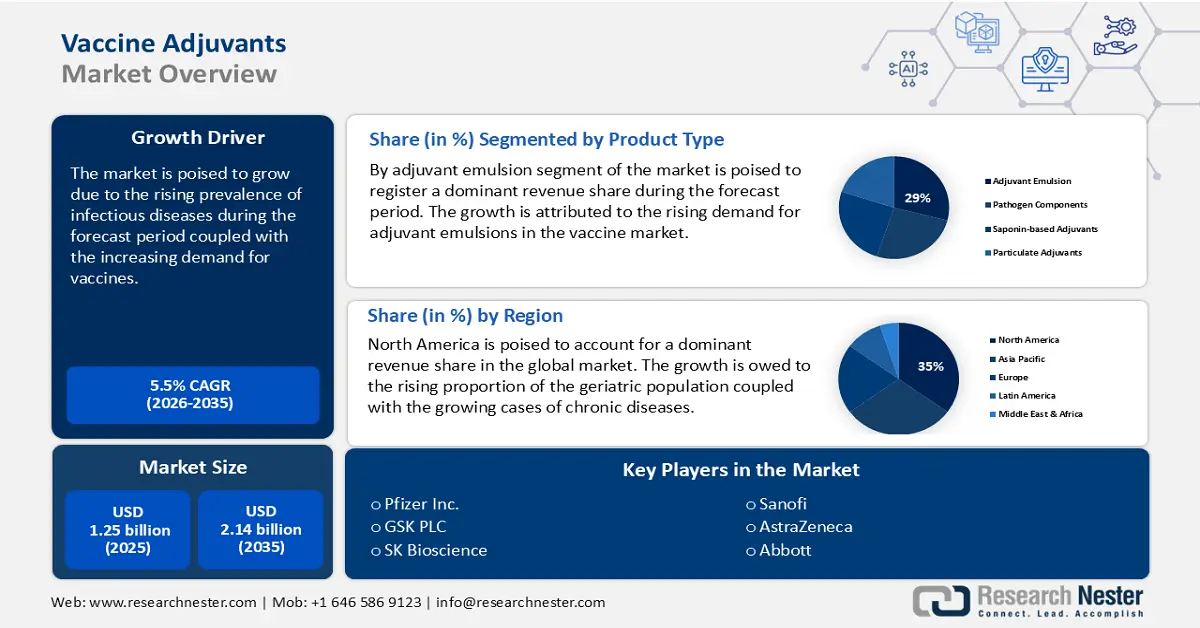
Vaccine Adjuvants Market size was over USD 1.25 billion in 2025 and is anticipated to cross USD 2.14 billion by 2035, growing at more than 5.5% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of vaccine adjuvants is assessed at USD 1.31 billion.
The growth of the vaccine adjuvants market can be attributed to the growing prevalence of infectious and allergy diseases such as HIV/AIDS, tuberculosis (TB), malaria, and neglected infectious diseases along with increasing demand for advanced and improved vaccines. NTDs encompass a diverse group of infections caused by parasites, bacteria, fungi, viruses, and other non-communicable pathogens. In addition, there were 1.6 million deaths from TB in 2021 (including 187 000 HIV-positive individuals). According to the World Health Organization, TB is the 13th leading cause of death and the second leading infectious killer in the world after COVID-19. Vaccine adjuvants are substances that improve the effectiveness of vaccines. The growing prevalence of infectious diseases has caused an increase in demand for more effective vaccines, which has in turn contributed to greater research and development of new vaccine adjuvants.
In addition to these, factors that are believed to fuel the vaccine adjuvants market growth of vaccine adjuvants include an increase in financial support from governments and private organizations for the development of vaccines and the growing demand for safe and effective immunization are expected to have a positive impact on the growth of the global vaccine adjuvants market. In addition, funding for vaccine research and infectious disease research is expected to enhance the lucrative prospects for vaccine adjuvants. For instance, to prevent epidemics, detect disease threats, and respond rapidly and effectively to epidemics, the US government announced a funding of USD 122 million for Indian medical research institutes in June 2022.
Key Vaccine Adjuvants Market Insights Summary:
Regional Highlights:
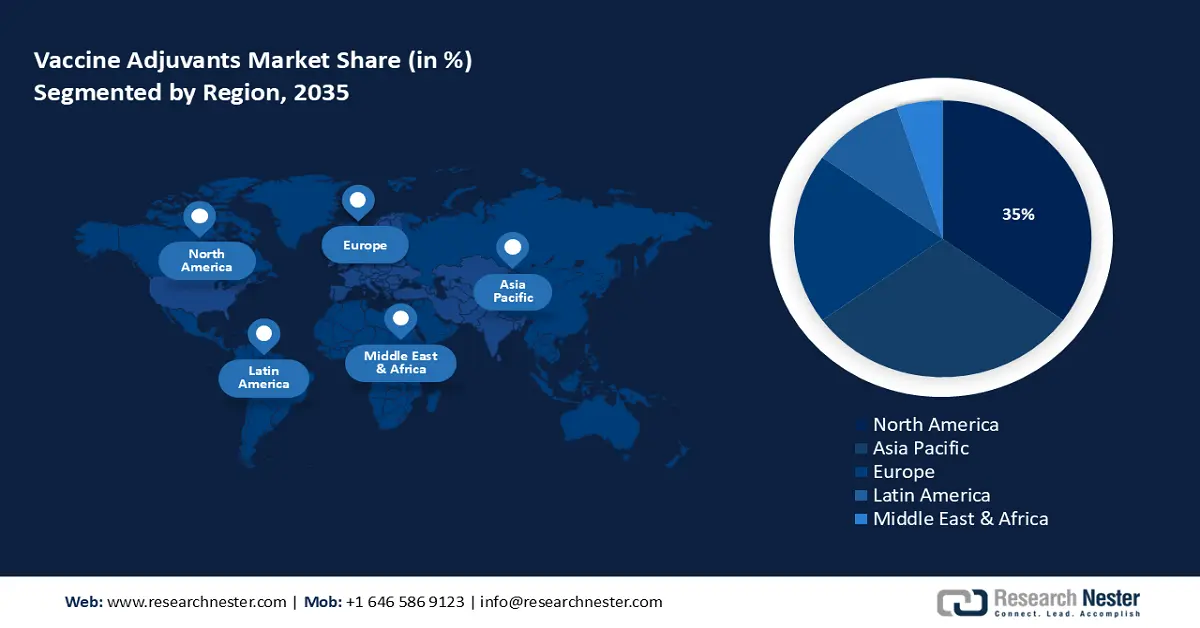
- North America vaccine adjuvants market will dominate more than 35% share by 2035, driven by a large elderly population and increasing prevalence of chronic diseases.
- Asia Pacific market will account for 24% share by 2035, attributed to increased investment in vaccine R&D and rising demand for combined vaccines.
Segment Insights:
- The intramuscular segment in the vaccine adjuvants market is expected to capture a 37% share by 2035, driven by its sustained vaccine release and reduced pain compared to other administration routes.
- The adjuvant emulsions segment in the vaccine adjuvants market is anticipated to secure a 29% share by 2035, influenced by their capability to enhance vaccine efficacy and rising immunization awareness.
Key Growth Trends:
- Rising Number of Government Initiatives to Promote Vaccination and Support the Development of New Vaccines
- Growing Cases of Seasonal Influenza
Major Challenges:
- Long approval process
- High cost of vaccine adjuvants
Key Players: Pfizer Inc., GSK plc, SK Bioscience, Sanofi, Abbott, Phibro Animal Health Corporation, Astrazeneca, Novavax, Inc., Agenus Inc., Astellas Pharma Inc.
Global Vaccine Adjuvants Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 1.25 billion
- 2026 Market Size: USD 1.31 billion
- Projected Market Size: USD 2.14 billion by 2035
- Growth Forecasts: 5.5% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (35% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, China, Japan, United Kingdom
- Emerging Countries: China, India, Brazil, Mexico, South Korea
Last updated on : 10 September, 2025
Vaccine Adjuvants Market Growth Drivers and Challenges:
Growth Drivers
- Rising Number of Government Initiatives to Promote Vaccination and Support the Development of New Vaccines - For instance, a National Strategic Plan (2021-2025) for vaccines and immunizations provide a vision for the future of the U.S. vaccine and immunization enterprise. US Healthy People 2030 initiative focuses on increasing vaccination rates to prevent infectious diseases. This initiative seeks to increase public awareness around the importance of vaccination and its role in the prevention of infectious diseases. Moreover, Indian government's vaccination initiatives such as Har Ghar Dastak and Jan Bhagidari Andolan have been successful in immunizing large sections of the population, especially in rural areas where access to healthcare is scarce. As a result, there has been a significant reduction in the prevalence of vaccine-preventable diseases.
- Growing Cases of Seasonal Influenza - Seasonal influenza causes between -3 million and 5 million severe illnesses and between 290,000 and 650,000 respiratory deaths per year, according to the World Health Organization. As the number of influenza cases increase, so does the need for more effective influenza vaccines. Vaccine adjuvants are substances added to vaccines to enhance their effectiveness, making them better able to protect against the virus.
- Increase in the Number of HIV/AIDS Cases - According to the Centers for Disease Control and Prevention (CDC), there were an estimated 1,189,700 HIV-positive individuals in the United States as of the end of 2019. HIV is most prevalent among black and African American people. The number of new HIV diagnoses among Black/African American people in 2020 was 42 percent (12,827).
- High Prevalence Chronic Diseases Such as Cancer have Prompted a Surge in the Demand for Vaccine Adjuvants- Vaccine adjuvants are used to enhance the body's immune response to the cancer cells, allowing the body to mount a stronger attack against the cancer cells. This can help to reduce the growth of the tumor and even eliminate it altogether. Thus, the rising prevalence of cancer is expected to drive vaccine adjuvants market growth. The World Health Organization estimates that cancer caused nearly 10 million deaths worldwide in 2020. Among the most common cancers are breast, lung, colon, and rectum cancers as well as prostate cancer.
- Increasing Development and Approval of Several COVID-19 Vaccines - For instance, Novavax COVID-19 Vaccine, adjuvanted for the prevention of COVID-19 in individuals 18 years of age and older, was approved by the United States Food and Drug Administration in July 2022 as an emergency use authorization (EUA).
Challenges
- Long approval process - Vaccines are heavily regulated, and the approval process can be lengthy and complex. This can delay the availability of new vaccines and even cause existing vaccines to be taken off the vaccine adjuvants market. The lengthy approval process can also make it difficult for new companies to enter the market.
- High cost of vaccine adjuvants
- Complexities associated with the development of vaccine adjuvants, as well as stringent regulatory requirements
Vaccine Adjuvants Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
5.5% |
|
Base Year Market Size (2025) |
USD 1.25 billion |
|
Forecast Year Market Size (2035) |
USD 2.14 billion |
|
Regional Scope |
|
Vaccine Adjuvants Market Segmentation:
Product Type Segment Analysis
The global vaccine adjuvants market is segmented and analyzed for demand and supply by product type into adjuvant emulsions, pathogen components, saponin-based adjuvants, particulate adjuvants and others. Out of these, the adjuvant emulsions segment is estimated to gain the largest vaccine adjuvants market share of about 29% in the year 2035. The increasing demand for adjuvant emulsions in the vaccine market owing to their capability to enhance the efficacy of the vaccine, as well as the rising awareness about the importance of immunization among the population are major factors driving the growth of this segment. For instance, a number of emulsion adjuvants have been used in commercially available influenza vaccines, such as MF59 and AS03, and their composition and performance have been extensively studied and reported. Moreover, increasing government initiatives to promote the use of adjuvant-containing vaccines to reduce the cost of the vaccine delivery system and enhance the overall efficiency of the vaccine are also contributing to the growth of this segment.
Route of Administration Segment Analysis
The global vaccine adjuvants market is segmented and analyzed for demand and supply by route of administration into intramuscular, subcutaneous, oral route and others. Out of these, the intramuscular segment is estimated to gain the significant vaccine adjuvants market share of about 37% in the year 2035. The intramuscular route of administration is becoming increasingly popular among the patient population owing to its ability to provide a more sustained release of the vaccine, thereby improving the efficiency of the vaccine. Research has shown that 30 to 50% of intended intramuscular injections succeed, with the remainder resulting in inadvertent subcutaneous drug placement. Additionally, intramuscular injections are considered to be less painful than other routes of administration, making them more desirable for patients. These factors are anticipated to create numerous opportunities for the growth of the segment in the coming years.
Our in-depth analysis of the global vaccine adjuvants market includes the following segments:
|
By Product Type |
|
|
By Route of Administration |
|
|
By Disease Type |
|
|
By Application |
|
|
By Application Category |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Vaccine Adjuvants Market Regional Analysis:
North American Market Insights
The market share of vaccine adjuvants in North America, amongst the market in all the other regions, is projected to be the largest with a share of about 35% by the end of 2035. The growth of the vaccine adjuvants market can be attributed majorly to the large population of elderly individuals and the increasing prevalence of chronic diseases, such as cancer, diabetes, and heart disease. Vaccine adjuvants enhance the immune system's response to the vaccine, resulting in a stronger and more effective response. With high recommendations for children’s vaccines, the demand for vaccine adjuvants in North America is expected to increase. For instance, United States standard recommendations include 10 vaccines for children between the ages of birth and 10 including hepatitis A (HepA); hepatitis B (HepB); RV; diphtheria, tetanus, and others. Additionally, increasing awareness about the importance of vaccinations, advances in vaccine technologies such as mRNA, and the growing adoption of preventive healthcare measures are likely to drive the growth of the market in this region. mRNA vaccines have revolutionized the speed of development and deployment of vaccines. mRNA vaccines are based on messenger RNA which provides instructions to cells to produce proteins that stimulate an immune response. These factors are anticipated to boost the vaccine adjuvants market growth during the forecast period.
APAC Market Insights
The Asia Pacific vaccine adjuvants market is estimated to be the second largest, registering a share of about 24% by the end of 2035. The growth of the market can be attributed majorly to the increased investment in vaccine research and development as well as the growing demand for combined vaccines. It was observed that a total of over USD 1 billion has been invested in Chinese vaccine research and development. A further 11.35 million doses of Covishield were exported by India in the year 2021. These doses were sent to 92 countries, including health workers and peacekeepers from the UN. Additionally, the rising prevalence of lifestyle-related diseases such as cancer and diabetes, as well as high prevalence of infectious diseases such as malaria and dengue, are expected to drive the demand for vaccines in the region, thus driving the growth of the vaccine adjuvant market in the Asia Pacific region.
Europe Market Insights
Europe region is likely to register significant growth till 2035. The growth of the market can be attributed majorly to the presence of a large number of vaccine manufacturers in the region and the increasing government support for vaccination programs. Additionally, Europe is also witnessing an increase in the prevalence of chronic diseases resulting in an increase in vaccine demand. Vaccines work by introducing a weakened or inactive form of a virus or bacteria into the body, prompting the immune system to produce antibodies and build immunity. Adjuvants are added to vaccines to enhance the body’s immune response and to reduce the amount of the active ingredient, or antigen, needed in a vaccine. This makes them more effective at combating chronic diseases and are anticipated to boost the vaccine adjuvants market growth during the forecast period.
Vaccine Adjuvants Market Players:
- Pfizer Inc.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- GSK plc
- SK Bioscience
- Sanofi
- Abbott
- Phibro Animal Health Corporation
- Astrazeneca
- Novavax, Inc.
- Agenus Inc.
- Astellas Pharma Inc.
Recent Developments
-
As the basis for their applications for regulatory approval, Sanofi and GSK plc intend to submit data from their booster and Phase 3 efficacy trials. The data they plan to submit is expected to include the full results of their Phase 3 efficacy trial of the vaccine, as well as data from their booster trial, which is designed to assess the efficacy of the vaccine after a booster dose.
-
GSK plc and SK Bioscience have submitted an application for a biologics license to the Korean Ministry of Food and Drug Safety for their SKYCovione after receiving positive Phase III clinical results. It is a candidate COVID-19 vaccine based on recombinant protein that has been adjuvant with GSK's pandemic adjuvant.
- Report ID: 4796
- Published Date: Sep 10, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Vaccine Adjuvants Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




