Sterile Injectable Drugs Market - Regional Analysis
North America Market Insights
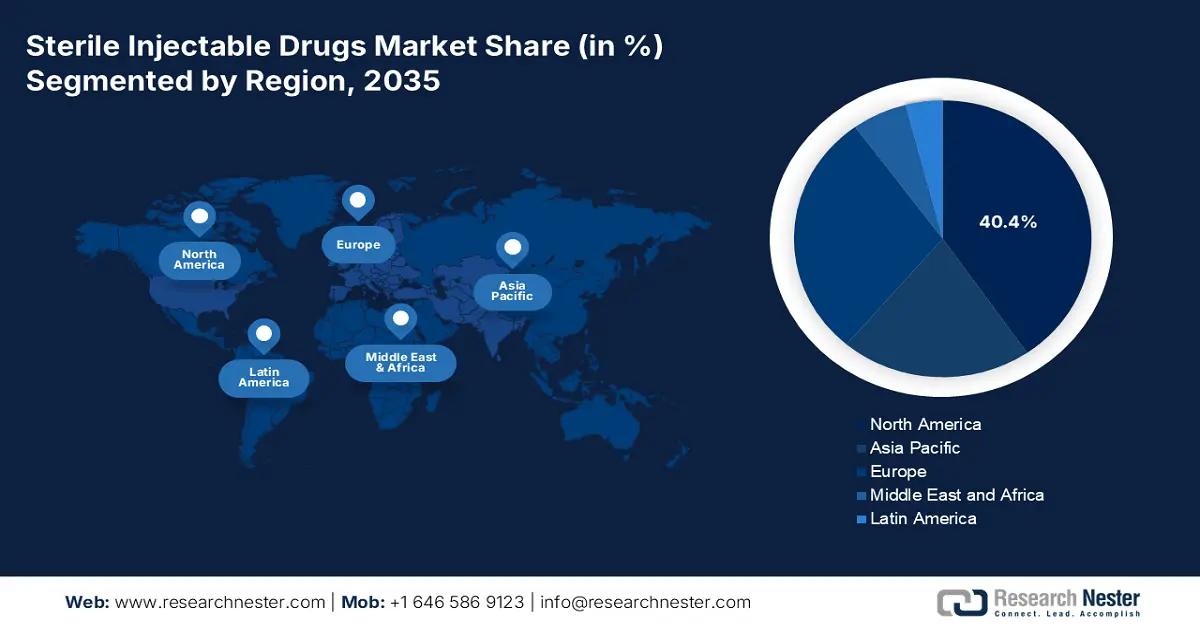
North America is the dominating region in the sterile injectable drugs market and is anticipated to accumulate a market share of 40.4% by 2035. High government spending and an expanded rate of medical coverage are one of the main growth-driving factors that accelerated the demand in the concerned market. the U.S. Centers for Medicare & Medicaid Services forecasts predict that in 2023, U.S. health care spending increased by 7.5% to USD 4.9 trillion, or USD 14,570 per person. Medicare coverage is expanded to ensure better coverage for the premium biologics like Keytruda, Humira, and many more, which has led to more accessibility for patients. The presence of advanced health care infrastructure and an oncology pipeline streamlined the diagnosis process and fast intervention, which raised the market demand for sterile injectable drugs in North America.
The U.S. sterile injectable drugs market is the largest in North America. The rise of chronic disease and the expansion of federal funding are the two leading factors that have raised the market performance of sterile injectable drugs. This resulted in high accessibility of the market, and a boost in the demand graph of sterile injectable drugs is experienced in the U.S. market. As per the report of the National Health Commission, monoclonal antibodies are relied on to intervene in oncology patients through chemotherapy or immunotherapy. In addition, innovations in drug-delivery systems like prefilled syringes and auto-injectors are improving patient adherence and safety. The regulatory environment is generally rigorous, although it also supports innovation by facilitating expedited approvals for critical therapies.
The sterile injectable drug market in Canada is flourishing, largely due to increased government spending on healthcare and the growing accessibility of biologic therapies for lifelong and rare diseases. The healthcare system in Canada is focused on enhancing patient outcomes and working to broaden its use of ready-to-use injectable formulations and innovative devices. The level of interest and investment in cancer care and immunotherapy is another significant aspect of growth in the market. Canada has a regulatory climate that supports the development of biosimilars that will make advanced treatments accessible and more affordable.
Asia Pacific Market Insights
The Asia Pacific market is emerging and is anticipated to hold a market share of 20.8% by 2035. Cancer incidence is expanding at a rate of twice comparison of the Western market, creating a high demand for sterile injectable drugs in the oncology department. Government spending accelerated to elevate the healthcare infrastructure in Asia Pacific, which resulted in an increased number of diagnoses and raised awareness within the market. Government-led initiatives for screening increased the number of chronic diseases, and the expansion of insurance coverage created a scope to leverage patient accessibility. Manufacturing of the drug is conducted through the implementation of local sourcing, that made Asia Pacific eligible to offer an affordable price range for the sterile injectable drugs, which has accelerated the demand for the market.
China is the largest regional market shareholder in the sterile injectable drugs market in Asia Pacific, driven by an increasingly older population, a greater prevalence of chronic diseases and infectious diseases, and by a focus on innovative biologics and immunotherapies. The ability of patients to access injectable drugs has improved as a result of the government's efforts to strengthen the healthcare infrastructure and expand insurance coverage. In addition, the regulatory environment is being strengthened in an effort to conform to international standards, stimulating domestic production and exports. The demand for ready-to-use injectables and advanced delivery devices/packaging for use in hospital and home care environments will also contribute to the growth of the market.
India market is growing rapidly as a result of a growing population, increasing prevalence of chronic diseases, and a growing need for advanced therapies such as biologics and vaccines. The Indian pharmaceutical sector is witnessing significant investment in healthcare facilities. The growing middle class and growing awareness towards developing new healthcare means access to sterile injectable treatments is growing. The growth of CMOs and an increasing market for generic injectables, and growing production capability and ability to sell treatments at lower price points. Regulatory changes made in government and agencies have encouraged faster drug approvals, which further enable reduced time to market for new sterile injectable products.
Europe Market Insights
The sterile injectable drugs market in Europe is currently demonstrating consistent demand growth, due in part to an aging population, increasing prevalence of chronic diseases, and increasing use of complex biologics and biosimilars. Furthermore, governments and insurance systems around Europe are investing in strategies to improve patient access to innovative injectable medicines. The door is also being opened for mixing sterile ingredients to create a sterile injectable in the outpatient or home setting. Various trade organizations are supporting this new change and cultural shift. Innovations in manufacturing technologies and adherence to consistent and definitive regulatory processes provide consistent quality in sterile injectable production. Finally, the trend towards sustainability and digital health solutions will help continue the growth of the market in Europe and around the globe.
The sterile injectable drugs market in France is consistently growing, supported by a robust healthcare system and high demand for advanced therapies. Part of this growth is due to government priorities to improve patient access to innovative treatment options and more government spending on healthcare. France also sees higher utilization of ready-to-use injectable products that increase safety and ease of use. Because of the government's significant investment in healthcare infrastructure, advanced medical treatments are now widely available in hospitals and clinics across the country. This accessibility fuels the need for injectable drugs used in a range of therapeutic areas.
With a strong pharmaceutical and healthcare system, Germany remains among the most significant markets for sterile injectable drugs in Europe. The demand for manufactured injectable biologics and biosimilars has increased as the comorbidities, due to the increased prevalence of chronic disease, and the aging population, increase. In addition, the regulatory environment in Germany provides for ease of innovation with a relatively uncomplicated process of providing early access to much-needed critical therapies. The growth of outpatient care and self-administration of medication via the increased reliance on auto-injectors and pre-filled syringes is also contributing to enhanced patient adherence.