Rubella Treatment Market - Regional Analysis
APAC Market Insights
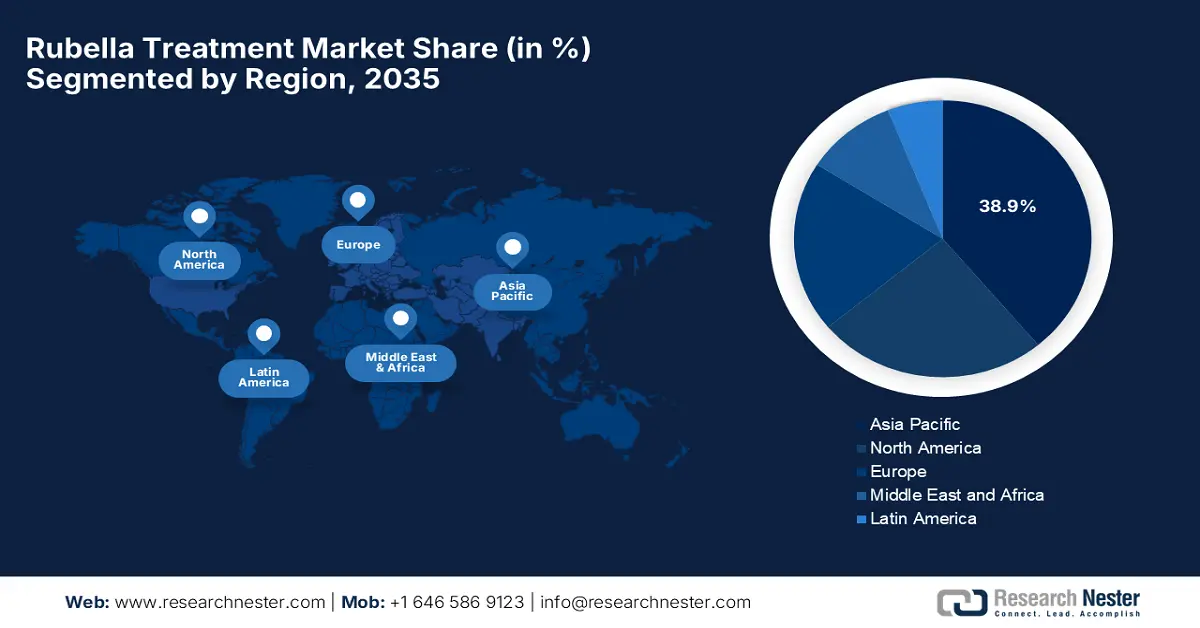
Asia Pacific is the dominant region in the rubella treatment market and is expected to hold the market share of 38.9% by the end of 2035. The market in the region is driven by the expanding government investments and maternal health policies. South Korea and Japan have higher vaccination rates. Further, South Korea and Malaysia are actively investing in maternal-fetal care via screening-linked treatment protocols supported by subsidized hospital programs. Multinational and regional players lead the market via localized production and public-private care models.
India is expected to lead the regional rubella treatment market on account of government support with the launch of multiple measles-rubella vaccination campaigns targeting children to increase immunization coverage and reduce the incidence of rubella. In March 2024, the country’s MoHFW noted that India has been honored with the prestigious Measles and Rubella Champion Award for its outstanding efforts in reducing measles and rubella cases through comprehensive vaccination campaigns and robust surveillance under the Universal Immunization Programme.
China holds a strong position in the Asia Pacific’s rubella treatment market, owing to the increasing vaccination efforts. For instance, in November 2022, the study by Vaccine reported that Beijing introduced a three-dose rubella-containing vaccine schedule, which is administered at 8 months, 18 months, and 6 years of age. In addition to this routine schedule, extra immunization efforts were directed toward high-risk groups. Besides, the study also revealed a wide variety of rubella virus strains circulating in the city, reinforcing the need for strong vaccine coverage and providing a promising opportunity for pioneers in this field.
North America Market Insights
The rubella treatment market in North America has a huge opportunity in the years ahead, which is driven by the robust public health framework, government reimbursement programs, and high prenatal care standards. For instance, in June 2022, GSK Plc reported that it had received US FDA approval for Priorix, its measles, mumps, and rubella (MMR) vaccine, for use in individuals aged 12 months and older, which will now provide US healthcare providers with an additional option to help protect patients against these contagious diseases.
The U.S. rubella treatment market is progressing steadily, supported by increasing maternal care programs and Medicaid or Medicare reimbursement reforms. Prenatal intervention demand related to rubella has grown, as rubella outbreaks have occurred among unvaccinated communities. The January 2025 article from CDC states that the rubella vaccine is given only as part of the MMR or MMRV combination vaccines, with two doses recommended for children, which should be first at 12 to 15 months and second at 4 to 6 years.
There is a huge exposure for the rubella treatment market in Canada, backed by the strong emphasis on vaccination programs, public health initiatives, and government agencies actively promoting immunization to control outbreaks. In March 2025, the country’s government reported that in 2025, the majority of measles cases occurred among individuals who were unvaccinated, accounting for 82% of reported cases, one dose (4%) or two or more doses (10%) of the measles-containing vaccine, highlighting gaps in vaccine coverage and the urgent need to increase awareness in the country.
Rubella Vaccine Recommendations from CDC
|
Category |
Recommendation |
|
Adults |
1 or 2 doses of MMR if no evidence of immunity; important for healthcare workers, travelers, and childbearing-aged women |
|
Pregnant Women |
Should NOT get MMR during pregnancy; vaccinate immediately after delivery if no immunity |
Source: CDC
Europe Market Insights
Europe is retaining its strong position in the rubella treatment market due to the presence of robust public reimbursement frameworks and government-backed maternal health programs. In July 2024, NIH analyzed rubella immunity among 7,937 pregnant women in Rome from 2021 to 2023, which found that 91% were immune while 9% were susceptible. Besides the WHO declaring rubella eliminated in Italy in 2022, the 9% susceptibility rate exceeds the National Plan's target of under 5% for women of childbearing age, which highlights the continued importance of preconceptional rubella vaccination, denoting a positive market outlook.
Germany is augmenting its leadership in the regional rubella treatment market due to increased maternal infection screening and a strong focus on prevention through vaccination, as there is no specific antiviral treatment for rubella once infection occurs. Besides the efforts to increase vaccination coverage and public awareness, which contribute significantly to managing the disease burden, they are also readily fostering a profitable business environment for the rubella treatment industry.
The U.K. is one of the most influential landscapes for the rubella treatment market, backed by a highly organized public health framework where the primary focus is on prevention through a robust national immunization program. The country’s government in September 2025 stated that MMR vaccine coverage remains high but shows a slight decline over recent years, wherein the first dose coverage is around 92% to95% and the second dose is between 84% to 89%. The UK continues to maintain strong surveillance and vaccination efforts, including plans to bring the second MMR dose forward.
UK Measles and Rubella Elimination Indicators
|
Year |
MMR 1st Dose Coverage (%) |
MMR 2nd Dose Coverage (%) |
Measles Cases |
Measles Incidence per Million |
Measles WHO Status |
Rubella WHO Status |
|
2021 |
93.8 |
86.5 |
2 |
0.00 |
Eliminated |
Eliminated |
|
2023 |
92.3 |
84.5 |
481 |
6.00 |
Eliminated |
Eliminated |
|
2024 |
92.3 |
84.5 |
3,681 |
51.3 |
Pending |
Pending |
Source: GOV.UK