Pancreatic Elastase Testing Market - Regional Analysis
North America Market Insights
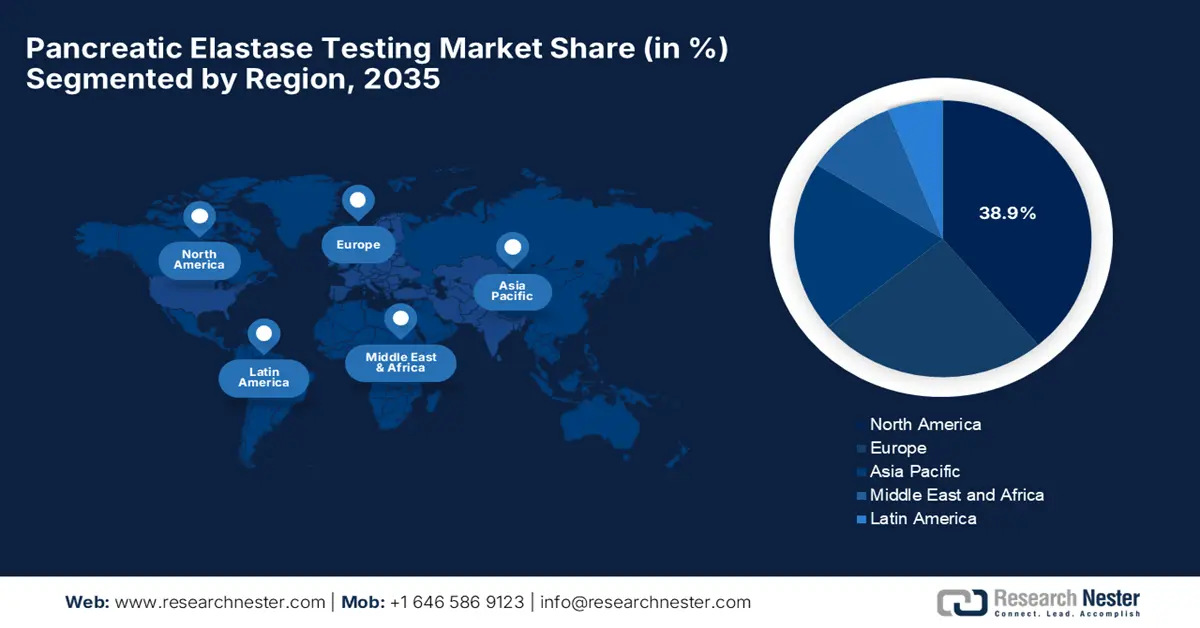
The pancreatic elastase testing market in North America is projected to register 38.9% of the share by 2035, owing to rising incidences of gastrointestinal disease and increasing digital test access. Various companies in the region are making strategic partnerships with public health agencies to accelerate the market uptake. In the U.S, the launch of home-based stool test kits is a major driving factor, wherein Geneoscopy in July 2025 reported U.S. FDA approval for an updated stool collection kit for its RNA-based colorectal cancer screening test, ColoSense, aiming to simplify at-home screening.
Canada in the pancreatic elastase testing industry is also set to witness staggering growth due to increased funding through provincial and federal initiatives. The government in the country is emphasizing early-stage diagnostic testing. For instance, in March 2025, Health Canada reported a bilateral agreement in collaboration with Quebec to invest a substantial USD 305 million in improving access to drugs for rare diseases. The funding will support early diagnosis, screening, and access to new and existing treatments as part of Quebec’s Action Plan on Rare Diseases, hence benefiting overall market growth.
Canada's National Strategy for Drugs for Rare Diseases (2023)
|
Funding Purpose |
Amount |
Duration |
Notes |
|
Total Investment in National Strategy |
Up to USD 1.5 billion |
Over 3 years |
To improve access and affordability of rare disease drugs |
|
To Provinces and Territories via Bilateral Agreements |
Up to USD 1.4 billion |
Part of USD 1.5 billion total |
For access to new/emerging drugs, existing drugs, early diagnosis, and screening |
|
To the Canadian Institutes of Health Research (CIHR) |
USD 32 million |
Over 5 years |
For rare disease research, diagnostic tools, and a clinical trial network |
|
To National Governance Structures (Health Canada Secretariat & Advisory Group) |
USD 16 million |
Over 3 years |
To support the implementation of the National Strategy |
Source: Health Canada
APAC Market Insights
The pancreatic elastase testing market in the Asia Pacific is projected to progress with the highest CAGR between 2026 and 2035, fueled by rising healthcare expenditure and the establishment of upgraded diagnostic infrastructure. In a plethora of countries, AI-powered diagnostic practices and tests based on stool analysis are considered to be the gold standard practice amongst healthcare practitioners. Additionally, the administrative bodies across the region’s countries are also giving preference to the early diagnosis and adoption of non-invasive testing for pancreatic diagnostics.
In India, the widespread expansion of private facilities for diagnosis in the country, schemes such as the National Health Policy, are emphasizing the early detection of chronic diseases, augmenting demand in the market. For instance, in May 2024, Cipla reported an additional investment of up to INR 26 crore (USD 3.13 million) in Achira Labs Private Limited through Optionally Convertible Preference Shares, in four tranches, which strengthens its position in the point-of-care (PoC) diagnostics space, supporting Achira’s efforts to develop and commercialize innovative medical test kits in the country.
Europe Market Insights
The pancreatic elastase testing market in Europe is predicted to register a notable share by the end of 2035 due to the presence of a robust framework for approvals and exponentially increasing digestive disorders. In May 2025, ALPCO reported that it had launched the Calprotectin Immunoturbidimetric Assay in Europe, which is an FDA-cleared assay, expanding its gastrointestinal diagnostic portfolio. The company also reported that the assay was certified in 2022 (IVDD), remarkably, assisting in diagnosing inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis, with high sensitivity (90.5%) and specificity (93.4%).
The market in Switzerland is portraying steady growth, owing to increasing collaborations between major firms and advancements in diagnostic technologies. In October 2024, BÜHLMANN Laboratories AG announced that it had partnered with Beckman Coulter to distribute its BÜHLMANN fPELA turbo assay, an automated test for quantifying pancreatic elastase in stool samples, on Beckman Coulter’s clinical chemistry analyzers. Therefore, such moves enhance laboratory workflow efficiency by integrating pancreatic elastase testing into routine automated platforms, hence denoting a positive market outlook.