Migraine Nasal Spray Market - Regional Analysis
North America Market Insights
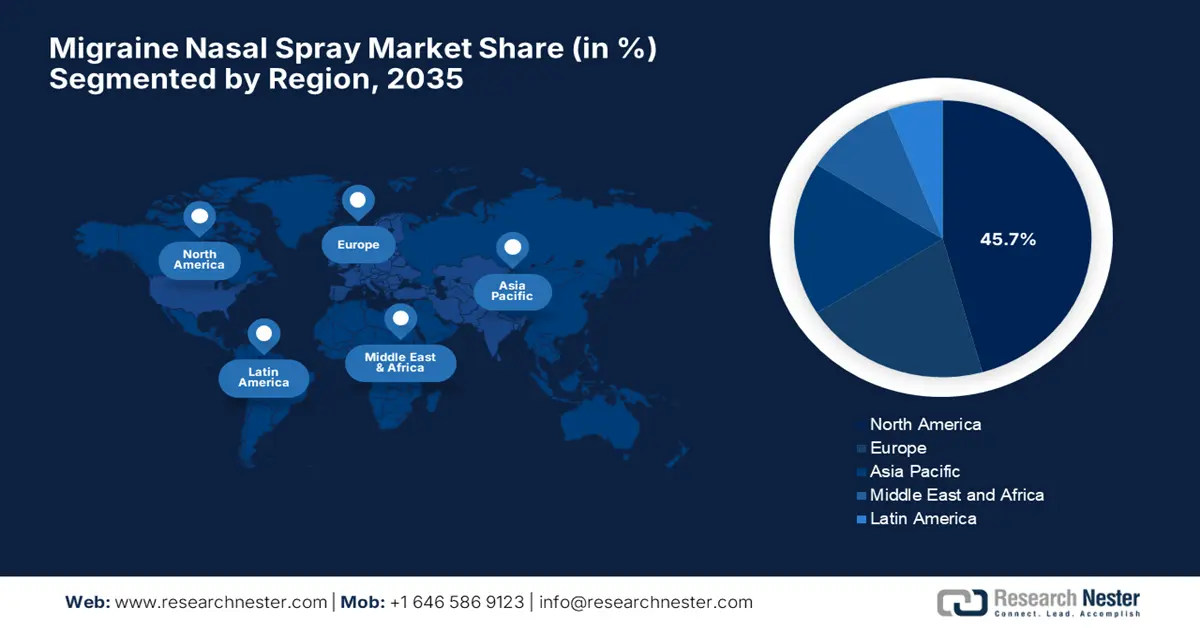
North America is predicted to garner the highest share of 45.7% by the end of 2035, owing to the payer reimbursement policies and fast-track U.S. FDA approvals. In March 2023, Vistagen was granted a U.S. patent for PH80, its investigational pherine-based nasal spray for the treatment of migraine, extending protection through at least 2040. The firm also underscored that PH80 offers a novel, non-systemic mechanism of action targeting the olfactory bulb and related brain pathways, hence suitable for overall market growth.
The U.S. is augmenting its leadership in the regional migraine nasal spray market, effectively propelled by increasing funding grants and a supportive regulatory environment. In 2024, the Headache & Migraine Competitive Grant Program reported that it allocates funds to innovative projects to improve migraine and headache care, thereby enhancing diagnosis, treatment, safety, and equity, excluding lab research and clinical trials. It also stated that in 2024, it awarded up to USD 250,000 for two-year projects, hence providing an encouraging opportunity for pioneers.
The market in Canada is witnessing astounding growth, bolstered by the burgeoning prevalence of migraine, government healthcare investments, and growing awareness of preventive healthcare. In this regard, in June 2025, Pfizer Canada, together with Migraine Canada and Migraine Quebec, reported that it had launched the second year of the Out of Office for Migraine Awareness campaign during Migraine Awareness Month, i.e., June. It also stated that the initiative encourages workplaces in the country to use an out-of-office alert for four hours, the typical duration of a migraine, to raise awareness and reduce stigma.
Funding and Economic Impact Data on Migraine Research and Care in the U.S
|
Metric |
Amount |
Notes |
|
NIH total budget (2023) |
Nearly USD 48 billion |
Total NIH budget for 2023 |
|
NIH funding for headache disorder research |
USD 59 million |
Allocated in 2023 |
|
Cap on indirect costs by NIH |
15% |
New policy limit on indirect costs |
|
Previous indirect costs by some universities |
Over 50% |
Used to negotiate for indirect costs |
|
The cost to the US economy from migraine. |
USD 78 billion |
Annual healthcare and lost productivity costs |
Source: MSC
APAC Market Insights
Asia Pacific is set to register the fastest growth in the migraine nasal spray market during the analyzed timeframe. The rapid upliftment is readily facilitated by neurological disease burdens and urban healthcare access. In December 2024, ARS Pharmaceuticals announced that its licensing partners in China, Japan, and Australia have filed for regulatory approval of neffy (epinephrine nasal spray) 2 mg in their respective countries, which offers a needle-free, easy-to-use, and compact alternative with a long shelf life for Type I allergic reactions, including anaphylaxis, which can cause a migraine.
China is gaining enhanced traction in the regional market owing to the increasing healthcare investments, rising awareness of migraine disorders, and the adoption of advanced treatment solutions. The urban pollution in cities and cases of stress escalation have also propelled the sale of migraine nasal sprays amongst the population. Besides, the market also benefits from the increasing volume of the patient population and emerging reimbursement policies; hence, all of these factors will responsibly uplift the market growth in the country.
India has become the target landscape for most investors involved in the migraine nasal spray market due to the increasing awareness about migraine as a serious health condition and the expanding healthcare infrastructure. For instance, in May 2023, Dr. Reddy’s Labs received approval from CDSCO to manufacture and market Sumatriptan Nasal Spray 10 mg/0.1 ml for the acute treatment of migraine attacks with or without aura, hence suitable for standard market growth.
Europe Market Insights
Europe in the migraine nasal spray market is expected to grow at a notable pace, extensively backed by a surge in diagnosis rates and an increasing aging population. The initiatives taken from the European Health Data Space have fueled the market access for launching novel formulations. In June 2023, Vistagen announced that the Patent Office in the region intends to grant a patent for its PH80 nasal spray, which is a rapid-onset investigational treatment for migraine, with protection extending to at least 2040, hence denoting a positive market outlook.
The market in Germany is also expected to grow significantly over the forecasted years, supported by government spending and favorable policies for the market players. Besides, the country’s decentralized models for healthcare are accelerating the reimbursement policies for advanced therapies. Further, the usage of telemedicine has also mushroomed in the post-pandemic era, bolstering the market growth, particularly in elderly patients.
In the UK, the National Health Service has expanded the facilities for medication for migraine treatment and increased collaborations between firms, which is readily bolstering success in the migraine nasal spray market. For instance, in January 2025, CNX Therapeutics expanded its European CNS portfolio by securing exclusive rights to market Sumatriptan Alginate Film, a novel migraine treatment, across major markets in the region, hence elevating the sector’s growth in the country.