Medical Foods Market Outlook:
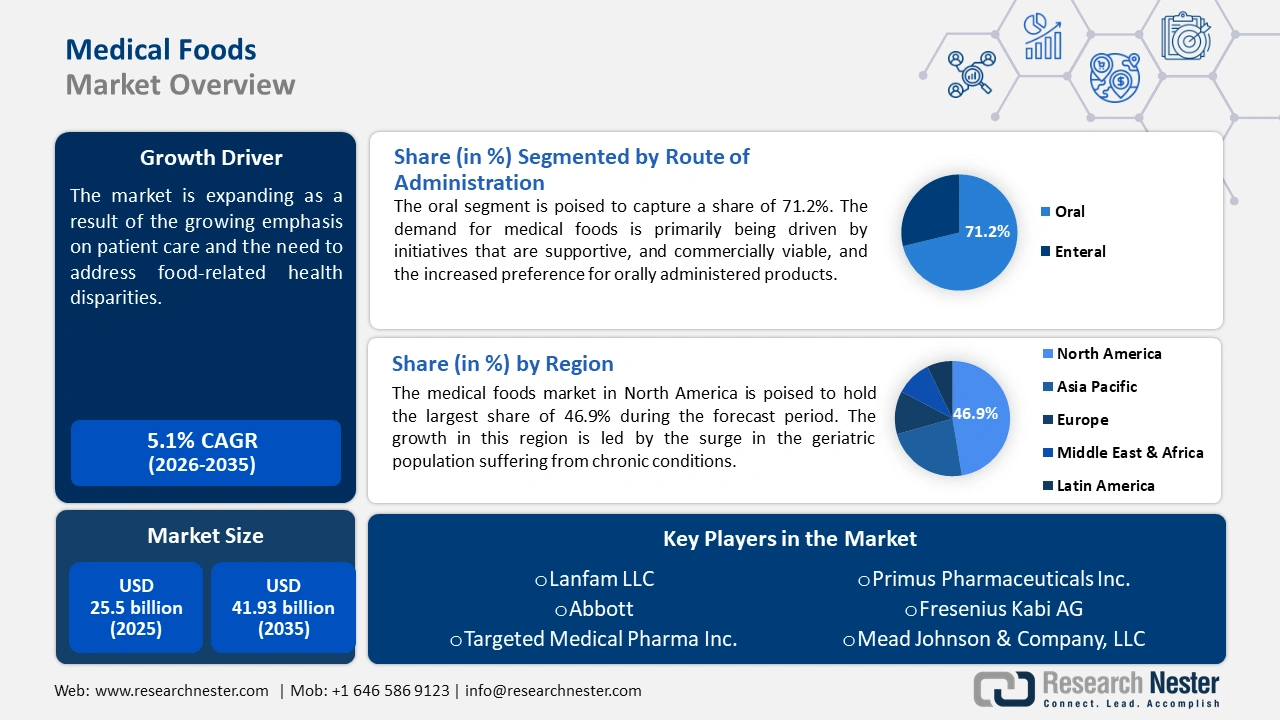
Medical Foods Market size was over USD 25.5 billion in 2025 and is projected to reach USD 41.93 billion by 2035, growing at around 5.1% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of medical foods is evaluated at USD 26.67 billion.
The growing emphasis on patient care and the need to address food-related health disparities have driven the demand for evidence-based nutritional medicines. Medical foods or foods for special medical purposes (FSMPs) as known in non-EU countries are specially formulated for the dietary management of patients with chronic diseases and are intended for use under medical supervision. Gaining traction owing to their potential disease management, medical foods are subject to general food legislation, such as good manufacturing practice (GMP), Codex Alimentarius, and the Orphan Drug Act under the FDA. The supportive regulatory frameworks have created opportunities for new entrants to capitalize on the rising demand and fostered the development of modern patient care.
A wide array of approved medical foods exists to help manage a range of medical conditions, including Alzheimer’s disease and HIV-associated enteropathy. EnteraGam contains serum-derived bovine immunoglobulin/protein isolate, which is being extensively used in treating inflammatory bowel disease (IBD), diarrhea-predominant irritable bowel syndrome, and HIV-associated enteropathy. Furthermore, Modulen IBD is a whole-protein nutrition formulation derived for treating the active phase of Crohn’s disease and Vivonex is used to manage severe gastrointestinal dysfunction. Stakeholders including the key players and medical practitioners are focused on clinical trials and developing promising therapies.