Gaucher Disease Drugs Market Regional Analysis:
North America Market Forecast
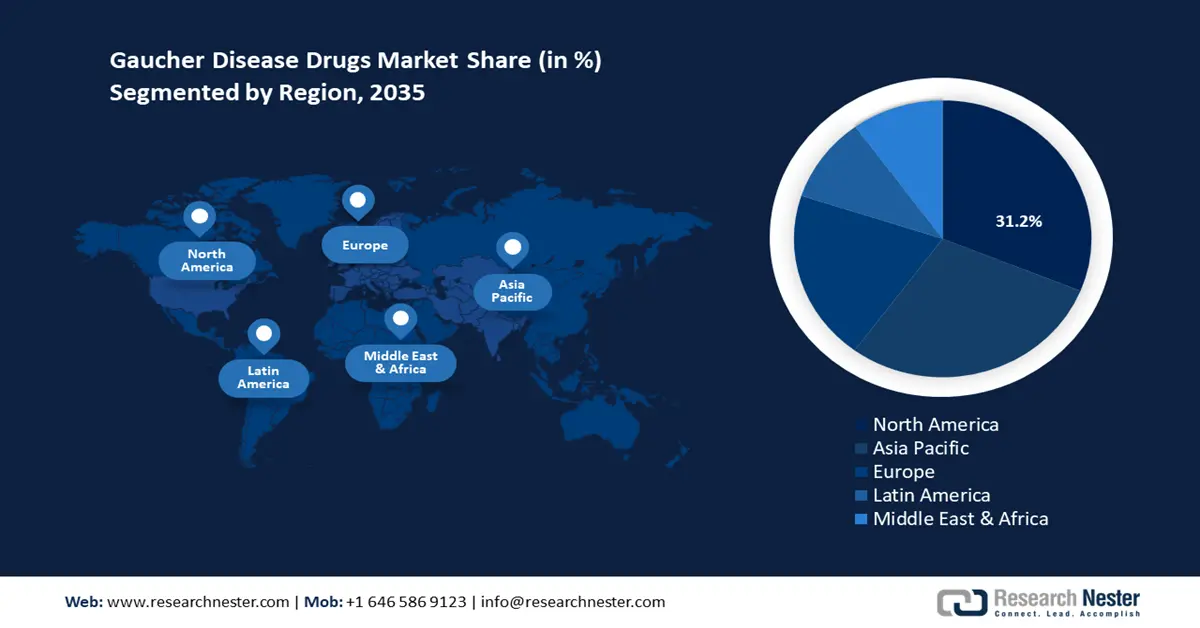
North America industry is likely to dominate majority revenue share of 31.2% by 2035. The market’s growth is attributed to the presence of a favorable regulatory ecosystem benefiting increasing clinical trials and drug approvals. The market’s profit share is led by the U.S. and Canada. Rising awareness of rare disease management and gaucher benefits the robust growth of the sector in the region. Additionally, access to advanced therapeutics supported by a well-established healthcare sector is positioned to continue the market’s profitable growth. For instance, in January 2020, AVROBIO announced FDA clearance for the new drug application for AVR-RD-02, i.e., an investigational gene therapy for gaucher disease treatment.
The U.S. holds the largest revenue share in the gaucher disease drugs sector. The market’s profitable growth curve is owed to a favorable regulatory environment supporting research on rare diseases. The U.S. Orphan Drug Act incentivizes pharmaceutical companies to develop drugs for rare diseases by offering tax credits and market exclusivity for a 7-year period. This creates a favorable regulatory environment for pharmaceutical and biotech firms to invest in research to find curative treatments for gaucher disease. Additionally, a well-established healthcare sector allows better management of conditions associated with gaucher such as anemia and bone disease. For instance, in March 2024, the FDA approved the first interchangeable biosimilars to Prolia and Xgeva to treat certain forms of osteoporosis that is a common side effect of gaucher disease.
Canada is poised to increase its revenue share in the global gaucher disease drugs market owing to expanding network of specialized treatment centers and a favorable healthcare system. The country’s universal healthcare improves access of treatments to wider demographics benefiting the market’s growth. Additionally, the domestic market in Canada benefits from government support to research efforts that have the potential to find new effective treatment for gaucher. For instance, in February 2024, the government invested USD 20 million to improve health outcomes of children diagnosed and living with rare diseases.
Domestic and global players eyeing the market in Canada can benefit from the government’s push to make drugs for rare disease more effective and affordable by leveraging government backed programs and funds. For instance, in March 2023, Canada launched the National Strategy for Rare Diseases to improve access and affordability of drugs effective for rare diseases and invested USD 32 million to advanced rare disease research in the country.
APAC Market Analysis
The APAC gaucher disease drugs market is poised to register the fastest growth in revenue share by the end of the forecast period. The growth is attributed to expanding healthcare infrastructure in the region coupled with rising awareness on the disease. Governments in APAC countries are boosting national frameworks on rare diseases, that is positioned to benefit the market. China, India, Japan, South Korea, and Australia lead the revenue share in APAC. A rising push for increasing government support for gaucher disease treatment is projected to benefit the market. For instance, in October 2024, the Lysosomal Storage Disorders Support Society of India forwarded a petition to the Government of India seeking sustainable treatment support for all patients diagnosed with Gaucher.
China is poised to register the largest share in the gaucher disease drugs market in APAC owing to promising research initiatives in the country led by CANbridge Pharmaceuticals Inc. For instance, in July 2023, CANbridge Pharmaceuticals Inc. announced the completion of the CAN103 phase 2 trial with the final patient completing their visit; CAN103 is a clinical-stage enzyme replacement therapy (ERT) that is showing promise for gaucher treatment.
Gaucher disease awareness is gradually picking steam in the country as efforts of genetic screening improve. The market’s future is promising in China as investments in improving health infrastructure and formulating policies to support rare diseases are positioned to benefit the gaucher disease drugs sector.
India is poised to increase its revenue share in the gaucher disease drugs sector in APAC. The large population in the country provides promising opportunities for pharmaceutical companies but a lack of awareness of gaucher disease leads to misdiagnosis or patients remaining undiagnosed. Recent trends are addressing the challenge and are positioned to benefit the market in India.
A major breakthrough is the inclusion of gaucher in the National Policy of Rare Diseases which is poised to reduce the economic burden of patients suffering from the disease, increasing patient footfall and treatment adoption in the country. For instance, in August 2024, the National Policy of Rural Diseases included gaucher Type 1 and Type 3 as diseases for which definitive treatment is available but face challenges to make optimal patient selection for benefit, very high cost, and lifelong therapy. This is positioned to create a favorable ecosystem in the country within the healthcare sector to support gaucher disease care.