Esketamine Nasal Spray Market Outlook:
Esketamine Nasal Spray Market size was valued at USD 27.11 billion in 2025 and is set to exceed USD 52.34 billion by 2035, registering over 6.8% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of esketamine nasal spray is estimated at USD 28.77 billion.
The primary factor that is set to drive the market for esketamine nasal spray and take it to the expected growth is the rise in the number of patients who require esketamine nasal spray. To illustrate a study made in 2023 almost 27.1% of patients on esketamine NS received remission and require this to cure their problem of depression. As Esketamine are utilized for treatment-resistant depression, and it is the patients’ first choice to have esketamine NS.
Additionally, another factor that will cast the esketamine nasal spray market to its expected growth is the several initiatives that were continuously taken by the world powers to combat the condition of depression. In 2019, WHO created the WHO Special Initiative for Mental Health (2019-2023): Universal Health Coverage for Mental Health to ensure the availability of quality and affordable care for mental health conditions in 12 priority countries to 100 million more people. Depression and self-harm/suicide are among the most important situations covered by WHO’s Mental Health Gap Action Programme (mhGAP) in 2023. The program focuses on assisting countries to prevalent services for people with mental, neurological, and material implementation disorders through attention given by health workers who are not experts in mental health. WHO has formulated concise psychological intervention manuals for depression that may be submitted by lay therapists to singles and groupsFurthermore, the Group Interpersonal Treatment for depression manual explains group treatment of depression. Lastly, the Thinking Healthy manual encircles the utilization of cognitive-behavioral treatment for ante-natal depression.
Key Esketamine Nasal Spray Market Insights Summary:
Regional Highlights:
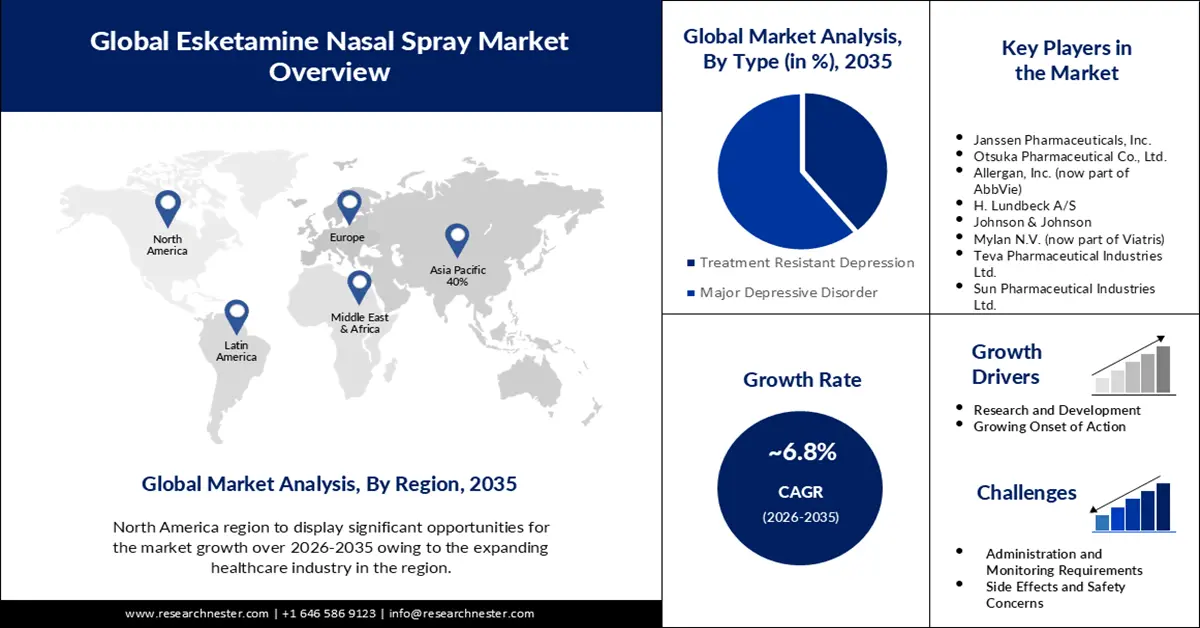
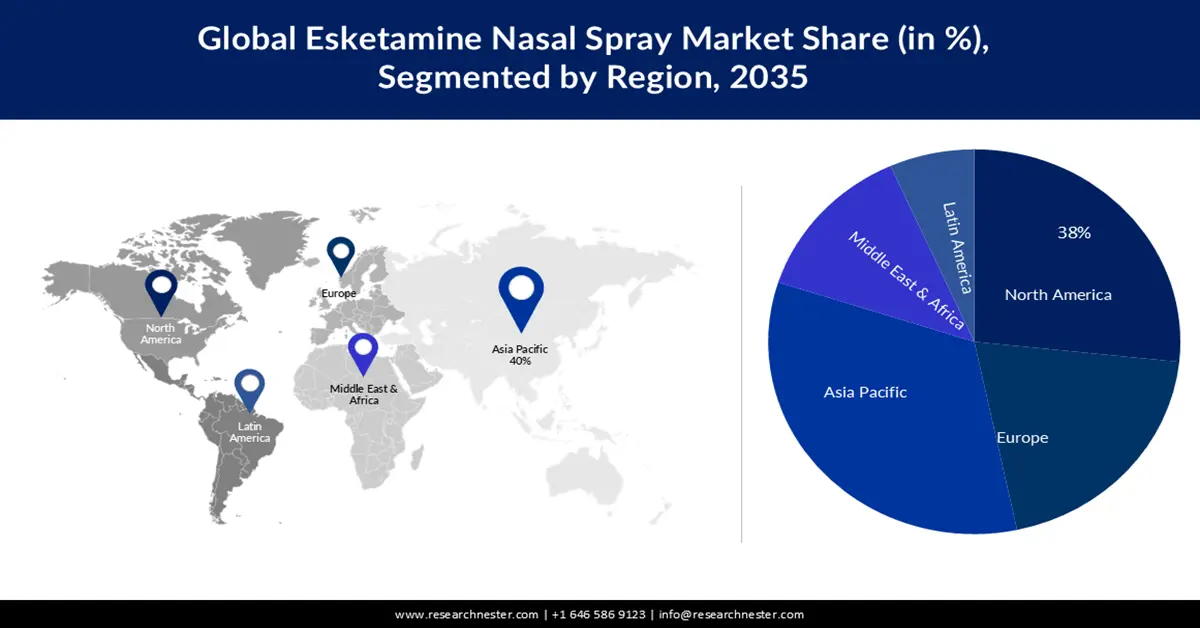
- North America is projected to secure a 38% share by 2035 in the esketamine nasal spray market, underpinned by the escalating prevalence of depression across the region.
- Europe is expected to witness substantial growth through 2035 as rising antidepressant consumption continues to accelerate mental-health-focused therapeutic adoption.
Segment Insights:
- The treatment-resistant depression segment is projected to claim about a 63% share by 2035 in the esketamine nasal spray market, propelled by expanding advancements in resistant-depression therapeutics.
- The adult segment is anticipated to capture a 59% share by 2035, supported by the widespread incidence of depression among adults globally.
Key Growth Trends:
- Increasing Prevalence of Mental Health Issues in the World
- Treatment Resistant Depression Therapies are Becoming Rapidly Popular Among Patients
Major Challenges:
- High Price of the Esketamine Drugs
- Lack of Standard Instructions in Diagnosing Patients
Key Players: Eli Lilly & Company, Bristol Myers Squibb Company, Novartis International AG, Bausch Health Companies Inc., Validus Pharmaceuticals LLC, Pfizer Inc., AbbVie Inc., Merck KGaA, Endo International Plc, MedKoo Biosciences, Inc., Janssen Global Services, LLC, Otsuka Pharmaceutical Co., Ltd., Biogen Inc., Sage Therapeutics, Inc.
Global Esketamine Nasal Spray Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 27.11 billion
- 2026 Market Size: USD 28.77 billion
- Projected Market Size: USD 52.34 billion by 2035
- Growth Forecasts: 6.8%
Key Regional Dynamics:
- Largest Region: North America (38% Share by 2035)
- Fastest Growing Region: Europe
- Dominating Countries: United States, Germany, United Kingdom, Japan, China
- Emerging Countries: India, Brazil, South Korea, Canada, Australia
Last updated on : 25 November, 2025
Esketamine Nasal Spray Market - Growth Drivers and Challenges
Growth Drivers
- Increasing Prevalence of Mental Health Issues in the World - In recent years, there has been rising confirmation of the significant role mental health acts in accomplishing international growth aims, as stated by the involvement of mental health in the Sustainable Development Goals. In 2020, the number of people living with stress and depressive disorders increased substantially because of the COVID-19 epidemic. Initial calculations demonstrate 26% and 28% development concurrently for stress and major depressive disorders in just one year. While efficient prevention and therapy choices exist, most people with mental disorders do not have availability to efficient care. Plenty of people also encounter disgrace, discrimination, and violations of human rights. Moreover, 1 in every 8 people, or 970 million people across the globe were living with a mental disorder, with stress and depressive disorders the most natural.
- Treatment- Resistant Depression Therapies are Becoming Rapidly Popular Among Patients - Esketamine increases depression indications in a majority of depressed people in clinical trials. Research recommends that untreated depression causes long-term brain harm and is a risk factor for dementia. Studies demonstrate that people with depression have up to 20% reduction of the hippocampus, a region of the brain critical for memory and learning. However, esketamine may counterbalance the detrimental impacts of depression. Animal studies show that links between brain cells reduce under chronic depression, but esketamine inverts this stress-associated transformation. Recognizing the root causes of the mental and behavioral health crises will need to take into consideration an extensive array of socioeconomic and health factors, and in many cases will signify handling much wider societal inequities. Moreover, the usage of psychedelic drugs along with esketamine is also increasing in patients facing depression.
- Increasing Research and Development in the field of TRD- Moreover, treatment-resistant depression is a field of continuous growth and research. For instance, Psilocybin is a classic psychedelic medicine that has charmed rising research interest over the past 10 years as a feasible therapy for mood, anxiety, and associated situations. Phase 3 trials in TRD are programmed to begin in 2023. Early proof recommends that single doses of psilocybin provided with psychological support influence quick betterment in depressive indications that persist for some weeks.
Challenges
- High Price of the Esketamine Drugs- The high cost of drugs that are utilized as treatment-resistant depression drugs like the esketamine is expected to hinder the esketamine nasal spray market growth over the forecast period 2026-2035. However, the high costs associated with the operation and maintenance of facilities in the sector will be a hindrance to the growth of the esketamine nasal spray market. 93.5% of adults with a material abuse disorder did not get therapy in the past year (2022). 10% of youth encircled by personal insurance did not have access to mental health services in 2022. For instance, Johnson & Johnson released its nasal spray depression treatment, termed Spravato, in March 2023, and the global price will be USD 500 for a 56 mg dose and USD 900 for 84 mg. Patients with treatment-resistant depression are letting the chance slip on possibly life-changing therapy with ketamine because systemic limitations in the public health system have made it expensive.
- Lack of Standard Instructions in Diagnosing Patients
- Overflow of Shapeless Information on This Field
Esketamine Nasal Spray Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
6.8% |
|
Base Year Market Size (2025) |
USD 27.11 billion |
|
Forecast Year Market Size (2035) |
USD 52.34 billion |
|
Regional Scope |
|
Esketamine Nasal Spray Market Segmentation:
Type Segment Analysis
The treatment-resistant depression segment in the esketamine nasal spray market is anticipated to grow massively during the forecast period and is expected to have a revenue share of around 63% by the end of 2035 in the market. The surge in the frequency of treatment-resistant depression and the existence of few treatment choices has further stimulated the market players to begin research and development activities in full bloom. For instance, an open-label, global study released on October 04, 2023, discovered that patients getting SPRAVATO (esketamine) CIII nasal spray for treatment-resistant depression (TRD) were 1.54 times as possibly to get remission after eight weeks than those cured with quetiapine extended-release (XR) at Week 8. The rising occurrence of treatment-resistant depression has created awareness concerning its therapy. Various market players are making progressed medications that can be managed through nasal and intravenous routes instead of the conventional oral route of administration. The increasing growth of novel therapeutics implemented for the therapy of resistant depression by market players to launch their novel materials is projected to lift the esketamine nasal spray market.
Patient Segment Analysis
The adult segment is estimated to hold 59% share of the global esketamine nasal spray market by 2035. The reason behind this growth is completely due to the prevalence of adult depression across the world. About 5% of adults internationally have depression. That number is likely even higher as not everyone who has depression obtains an official diagnosis. In fact, according to the study' 50% of mental health problems are established by age 14 and 75% by age 24. Young adults 18 to 25 years old are at the highest risk of a major gloomy episode per a report from the National Institute of Mental Health. Most people take time to come to terms with nerve-racking events, like mourning or a relationship failure. When these nerve-racking events appear, the risk of becoming depressed is enhanced if an individual stops meeting with friends and family and tries to work with their issues on their own.
Our in-depth analysis of the global esketamine nasal spray market includes the following segments:
|
Type |
|
|
Application |
|
|
Patient |
|
|
Prescription |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Esketamine Nasal Spray Market - Regional Analysis
North American Market Insights
North America industry is estimated to hold largest revenue share of 38% by 2035. The major factor that is expected to boost the esketamine nasal spray market growth in the region is the increasing prevalence of depressed Americans. A calculated 2 million times each year, people with severe mental health situations are jailed or imprisoned in the U.S. Women are inordinately influenced at twice the rate as men. For this population, detention may worsen pre-existing indications. In fact, while the percentage of U.S. adults getting mental health therapy rose from 19.2% in 2019 to 21.6% in 2021, 42% of U.S. adults with an identifiable situation reported in 2023 that they could not afford to avail the therapy they required. A trio of new studies paints a gloomy picture of how overdose deaths, depression, and obstacles to care are burdening heaviest on unprivileged and minority people - and are arranging to broaden health discrepancies as the U.S. appears from the pandemic.
European Market Insights
The esketamine nasal spray market in the Europe region is poised to show a notable growth in the upcoming years. The consumption of antidepressant drugs (AD) and esketamine nasal spray has grown drastically in the last two decades in the Europe region The implementation of antidepressants grew by almost two and a half times from 2000 to 2020 in 18 European countries, in line with the Organization for Economic Cooperation and Development (OECD) data. The average antidepressant ingestion around 18 European countries was 30.5 DDD per 1,000 people per day in 2000 increasing to 75.3 DDD in 2020, a 147 per cent rise. The European Parliament has always been an advocate of the encouragement of good mental health and keeping mental health at the heart of EU managerial. Its Subcommittee on Public Health (SANT) is recently developing its initiative report on mental health.
Esketamine Nasal Spray Market Players:
- Eli Lilly & Company
- Company Overview
- Business Planning
- Main Product Offerings
- Financial Execution
- Main Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Bristol Myers Squibb Company
- Novartis International AG
- Bausch Health Companies Inc.
- Validus Pharmaceuticals LLC
- Pfizer Inc.
- AbbVie Inc.
- Merck KGaA
- Endo International Plc
- MedKoo Biosciences, Inc.
Recent Developments
- The Massachusetts General Brigham (MGB) led a new study that matched subanesthetic intravenous ketamine to electroconvulsive treatment (ECT) for the therapy of non-psychotic, treatment-resistant depression. The outcomes are released in the New England Journal of Medicine. In a clinical trial of 403 patients investigators discovered that 55 percent of those who got ketamine therapy suffered a constant betterment in depressive symptoms without major adverse impacts.
- Otsuka Pharmaceutical Co., Ltd. (Otsuka) and Mindset Pharma, Inc. (Mindset) confirmed that they have come into a conclusive planning agreement according to which Otsuka will obtain Mindset for approximately CAD 80 million in an all-cash deal. This agreement has been performed through Otsuka America, Inc. (OAI), a wholly-owned subsidiary of Otsuka. The Otsuka and Mindset boards of directors have accepted the deal. The purchase is anticipated to be completed during the fourth quarter of 2023, subject to needed techniques.
- Report ID: 5318
- Published Date: Nov 25, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Esketamine Nasal Spray Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




