Dystonia Drugs Market Outlook:
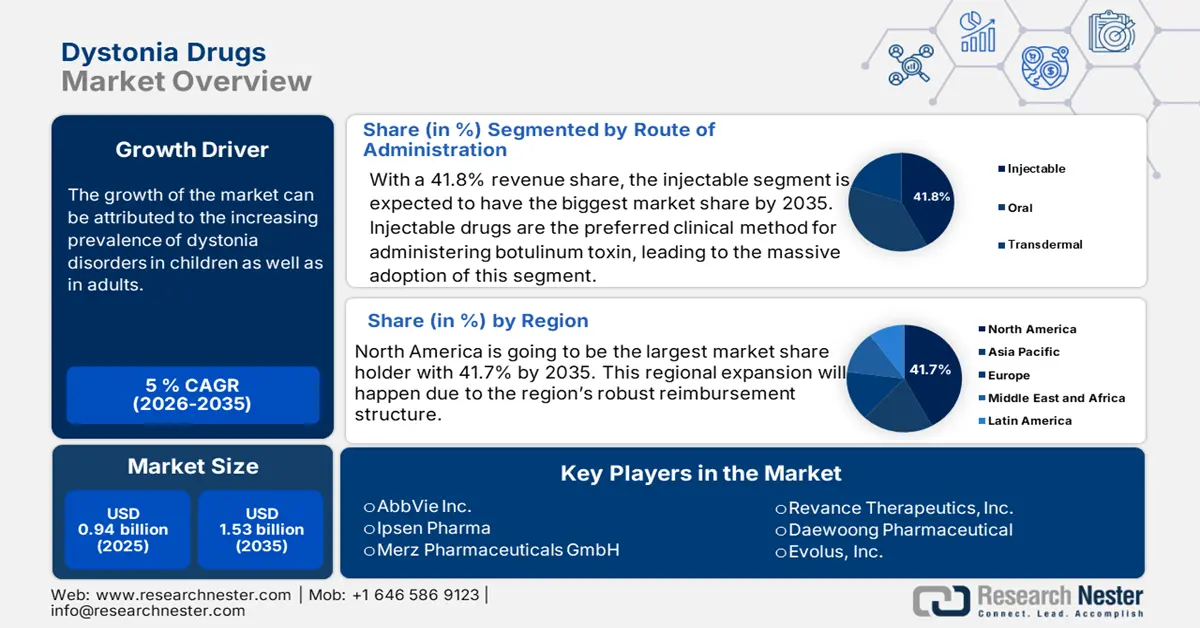
Dystonia Drugs Market size was valued at USD 0.94 billion in 2025 and is projected to reach USD 1.53 billion by the end of 2035, rising at a CAGR of approximately 5% during the forecast period (2026–2035). In 2026, the industry size of dystonia drugs is evaluated at USD 0.99 billion.
In 2023, the National Institutes of Health published a report stating that dystonia is 3rd most prevalent movement disorder, affecting 500,000 children and adults in North America. This surge in patient volume has resulted in a consistent increase in the active pharmaceutical ingredient supply and finished drug production. The botulinum toxin API, manufactured in various countries such as Germany, the U.S., and Ireland, is strictly regulated due to its biological origin and is categorized as a Schedule A substance under the FDA and EMA.
The trade dynamics of the drug are significantly shaped by the worldwide movement of high-value biologics, mainly botulinum toxin products. The product mainly originates from Germany, the UK, and Ireland, and requires strict cold chain logistics. Additionally, the largest import market is the U.S., becoming hugely exposed to altering policies such as tariffs on imports. On the other hand, oral medicines rely heavily on raw materials from China and India. This means that any issues or policy changes in these countries result in a lack of supply everywhere. To eradicate the chances of any disruptions, market players are giving attention to adequate storage facilities and backup suppliers.