Duchenne Muscular Dystrophy Treatment Market - Regional Analysis
North America Market Insights
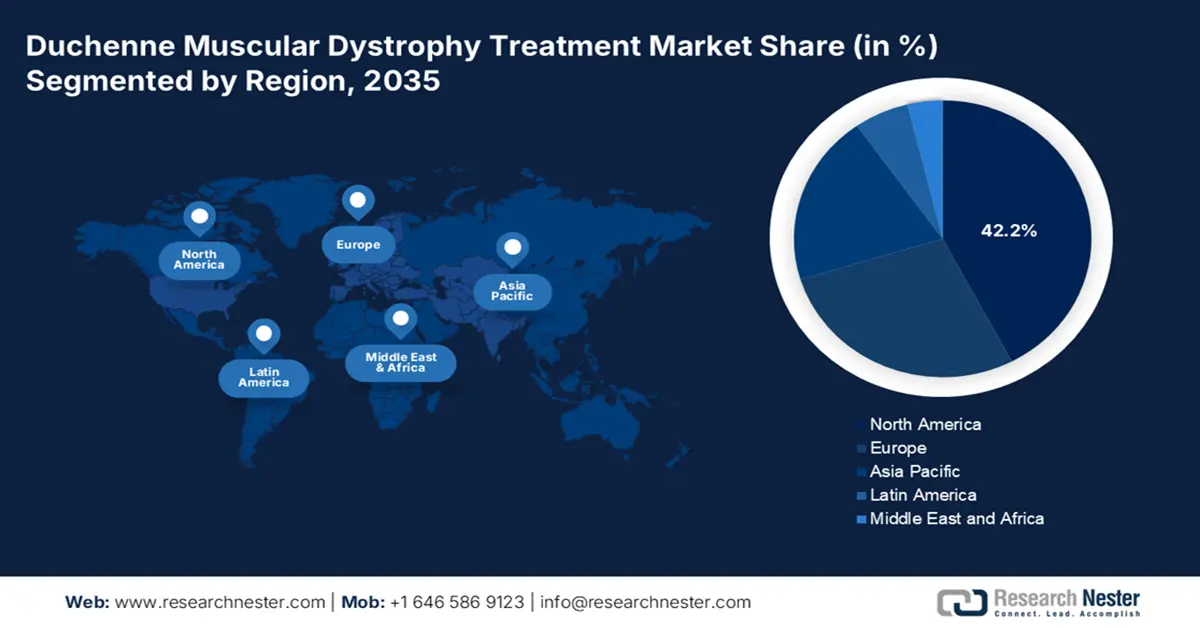
North America is anticipated to attain the largest share of 42.2% in the duchenne muscular dystrophy treatment market throughout the analyzed timeframe. The dominance is based on the robust pipeline of innovative therapies and broadened insurance coverage, which enhances patient access to advanced care. As per the CDC report in January 2025, nearly 14 in 100,000 males are affected by duchenne muscular dystrophy. Rising patient pool and healthcare advancements make North America a leader in therapeutic development and commercialization.
The U.S. dominates the regional Duchenne muscular dystrophy treatment market due to the substantial public and personal spending. As per the Rare Disease report in August 2025, fewer than 50,000 people in the U.S. have this disease. This is a genetic disease and mostly occurs at a young age. Market demand is fueled by strong patient advocacy groups like Parent Project Muscular Dystrophy (PPMD), which push for accelerated access and favorable insurance policies, ensuring that approved therapies reach the patient population despite annual costs.
Birth Prevalence and Diagnosis Rates of Duchenne Muscular Dystrophy
|
Year Range |
Region |
Birth Prevalence (per 100,000 male live births) |
Diagnosis Notes |
|
2020–2024 (approx) |
Global |
15.1–19.5 |
Consistent with historic rates; ranges approximately 1 in 3500 to 1 in 5000 male live births |
|
2023 |
United States |
2 per 10,000 males |
Diagnosis tends to occur before age 5 with improved genetic screening and newborn screening programs |
|
2022 |
Canada |
4.8 per 100,000 |
Prevalence consistent with rare disease profile; diagnostic advances underway |
Sources: NLM June 2022, NLM July 2023, NLM February 2022
APAC Market Insights
Asia Pacific is expected to achieve the highest growth rate in the duchenne muscular dystrophy treatment market by the year 2035. The increasing incidence of DMD, is the key driver for the region's outstanding spread in this segment. Furthermore, initiatives by governments in terms of rare diseases are also driving adoption in this segment. Key trends are the growth of newborn screening programs across countries such as South Korea and Taiwan, and strategic entry by multinational players through tie-ups with domestic manufacturers to enhance affordability and reach.
The China Duchenne muscular dystrophy treatment market is experiencing rapid growth on account of significant progress in cell-based research and development. Testifying to the same, the National Medical Products Administration (NMPA) approved DMD therapies in 2023. As per the NLM report in June 2022, the mean age at diagnosis is approximately 4.3 years, comparable to that in the U.S., and 71.3% of patients aged 5 and above use corticosteroids, a higher rate than in several Western countries. Telemedicine has emerged as an effective care model during the pandemic, highlighting the need for collaborative governmental and non-governmental efforts to improve drug accessibility for patients in China with DMD.
Europe Market Insights
The Europe duchenne muscular dystrophy treatment market is exhibiting robust growth to gain the 2nd largest revenue share by 2035. This is a result of favorable government initiatives and improved national healthcare policies. In this regard, based on the European Commission report in February 2025, European member states received €18.75 million to improve the national plan for rare diseases, which includes DMD. Further, the region is also benefiting from cross-border data sharing and national awareness programs.
Germany is leading the Europe duchenne muscular dystrophy treatment market with a huge public and private capital investment. According to the NLM report of August 2025, duchenne muscular dystrophy has a prevalence of 14.85 to 18.91 per 100,000 males aged under 40 years in 2021. The disease management programs (DMPs) of the country have enhanced the results of the patients through standardized care guidelines, and the healthcare costs extended to an average of €41,888.70 per patient each year in advanced stages, with more medical aids and interventions. The leadership of the nation is complemented by the early implementation of the cutting-edge therapies for rare disease fund in 2023.