Cutaneous T-cell Lymphoma Treatment Market - Regional Analysis
North America Market Insights
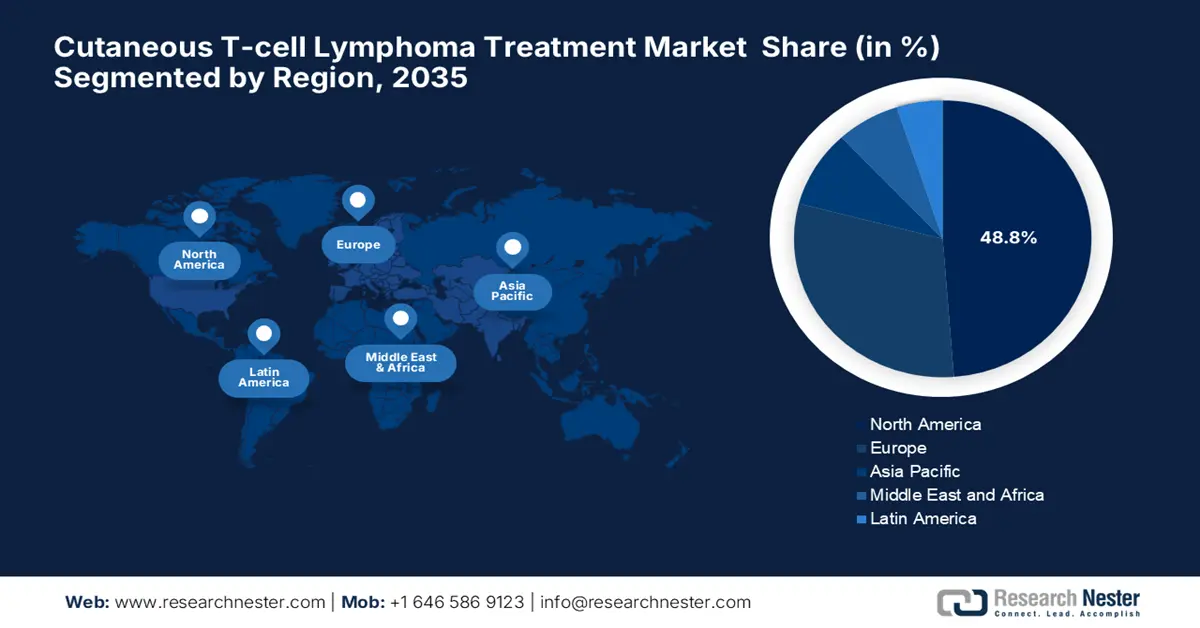
North America is expected to dominate the global cutaneous T-cell lymphoma treatment market with a share of 48.8% during the assessed time frame. The region benefits from an advanced healthcare infrastructure and robust R&D investments. The U.S. leads the landscape with maximum regional revenue, owing to the supportive governing bodies. Besides, by 2025, the region had 73 NCI-designated cancer centers that offer multidisciplinary CTCL care, denoting a profitable business opportunity. Furthermore, a majority portion of oncology practices in North America are emphasizing AI-based histopathology, solidifying the region’s dominance over this sector.
The U.S. is maintaining its strong dominance over the regional cutaneous T-cell lymphoma treatment market, efficiently backed by reimbursement coverage expansions and robust R&D investments. Exemplifying this, till February 2025, Citius Pharma alone invested more than USD 90 million in upfront purchase, development, pre-commercial efforts, and spinout of its revolutionary therapy LYMPHIR. The company further targeted the addressable U.S. market with approximately 3,000 CTCL patient pool that consists of a USD 400 million revenue opportunity.
There is an immense exposure for the Canada cutaneous T-cell lymphoma treatment market, which is backed by the substantial federal and provincial healthcare allocations and grants. In this context, in January 2025, the Governments of Canada and the province of Ontario collaboratively decided to invest more than USD 535 million over three years under the National Strategy for Drugs for Rare Disease (DRD) agreement. This funding was specifically intended to improve access to new and existing drugs, early diagnosis, and screening programs for residents of Ontario living with rare diseases.
Analysis of Overall and Cause‐Specific Mortality of CTCL in the U.S. (2025)
|
Metrics |
MF |
SS |
pcALCL |
SPTCL |
|
Patients diagnosed |
7957 |
272 |
1452 |
205 |
|
Incidence (per 1,000,000) |
6.1 |
0.21 |
1.1 |
0.16 |
|
Patient deaths |
1474 |
120 |
470 |
69 |
|
Follow‐up in months (median, range) |
66, 0-227 |
26, 0-216 |
66, 0-226 |
48, 0-218 |
|
2‐year OS, % |
93 |
67.7 |
88 |
79 |
|
5‐year OS, % |
83.6 |
38.4 |
79.9 |
71.3 |
Source: NLM
APAC Market Insights
Asia Pacific is likely to showcase the fastest growth in the global cutaneous T-cell lymphoma treatment market with an estimated CAGR of 7.6% from 2026 to 2035. The rigorous progress is a result of rising disease awareness, biosimilar adoption, and government-backed healthcare reforms. Japan is dominating in this region with the aspect of fast-track approvals and reimbursement coverage for novel therapies. On the other hand, South Korea’s CAR-T research and Malaysia’s telemedicine aspect provide a strong opportunity for players to capitalize on the region’s merchandise.
China commands the regional cutaneous T-cell lymphoma treatment market on account of accelerated NRDL inclusions of novel biologics. In addition, the domestic CAR-T trials and AI-based diagnostics adoption are gaining traction, which further secures steady progress for the country in this field. As evidence, in November 2022, the results from a multicenter and retrospective study conducted upon CD30‐positive lymphoma-afflicted patients in China showed a promising potential of Brentuximab vedotin (BV). The evaluation of dosage from August 2020 to September 2022 recorded 77.2% and 79.9% progression‐free survival (PFS) and overall survival (OS) rates among the study cohort after a median follow‐up of 11 months.
India is presenting lucrative opportunities for the cutaneous T-cell lymphoma treatment market with supportive government schemes and notable accessibility gaps. In this regard, the National Policy for Rare Diseases recorded a milestone in this category by offering a coverage of USD 56929.7 for the treatment of 63 rare diseases at 14 designated Centres of Excellence, reducing the financial burden of afflicted patients. Such initiatives inspire a greater volume of targeted or high-risk individuals to enroll for advanced therapy initiation, ultimately fostering lucrative opportunities for both domestic and foreign companies in this sector.
Europe Market Insights
Europe is representing consistent growth in the cutaneous T-cell lymphoma treatment market with the presence of favorable reimbursement policies and cross-border collaborations. Germany is the leading country in this landscape, holding a 32.6% of regional share owing to its strong captivity on pharmaceutical advances. Besides, the European Union (EU) undertook a Cancer Mission Initiative, which allocated a considerable fund to rare cancer research, including CTCL. Furthermore, the current innovations in CAR-T and targeted therapy development reflect its commitment towards establishing a progressive environment for the merchandise.
Germany shows a greater potential in the cutaneous T-cell lymphoma treatment market, effectively attributed to strategic pricing and yearly expenditure on advanced therapeutics. The Pharmaceutical Market Reorganization Act in the country imposes value-based pricing, which enhances patient access to required biologics. Besides, the country’s public and private spending on healthcare accounted for 12.8% of the national GDP in 2021 alone, with more than USD 8.5 billion in the pharmaceutical industry-based R&D expenditure. These figures indicate the presence of lucrative revenue opportunities in Germany.
The U.K. is offering a reliable business environment for the cutaneous T-cell lymphoma treatment market, which is empowered by pioneers with advanced drug development technologies and a strong emphasis on clinical trials. Additionally, the National Health Service (NHS) leverages the volume of capital influx through its Cancer Vaccine Launchpad, benefiting the pharma innovators in this sector. Besides, the NICE-imposed flexible pricing for orphan drugs expands patient access to cutting-edge therapeutics, whereas Genomics England is strengthening consumer trust by enabling real-world evidence.
Cost of Treating MF/SS by Stage in Public Hospitals in Spain (2024)
|
Stage |
Per-Patient Annual Cost (in USD) |
Share of Total Cost (in %) |
|
Stage I |
14154.2 |
81 |
|
Stage II |
27836.4 |
7 |
|
Stage III |
45914.1 |
6 |
|
Stage IV |
86150.2 |
6 |
|
Total National Cost |
92725421.2 |
100% |
Source: NLM