Cryptococcal Antigen Lateral Flow Assay Test Market Outlook:
Cryptococcal Antigen Lateral Flow Assay Test Market size was valued at USD 785.21 million in 2025 and is expected to reach USD 1.15 billion by 2035, expanding at around 3.9% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of cryptococcal antigen lateral flow assay test is evaluated at USD 812.77 million.

The increase can be ascribed to the rising prevalence of cryptococcal infection worldwide as well as the growing demand for accurate and timely diagnostic instruments. An estimated 15% of HIV-related deaths worldwide are attributed to it, with three-quarters of those deaths taking place in sub-Saharan Africa. An estimated 223,100 cases of cryptococcal meningitis lead to 181,000 deaths annually among people living with HIV. Early detection of cryptococcal infection using these tests enables timely treatment and better patient outcomes. Furthermore, lateral flow tests can be used in resource-constrained environments without complex laboratory equipment because they are frequently portable and simple to operate. For example, the flow test for cryptococcal antigen has shown excellent sensitivity and specificity in identifying cryptococcal antigen in serum and cerebrospinal fluid (CSF) samples.
Increased approvals for the cryptococcal antigen lateral flow assay tests are also driving the growth of the market. For instance, a dipstick-test lateral flow assay (Immuno-Mycologics, USA) for the semi-quantitative detection of cryptococcal antigen was authorized by the US Food and Drug Administration (FDA) in 2011. This test has the potential to be more advantageous than conventional techniques due to its quick turnaround time, simplicity of use, and reagent stability at room temperature. The World Health Organization suggests using this assay to check for cryptococcal antigen in HIV-positive individuals.
Key Cryptococcal Antigen Lateral Flow Assay Test Market Insights Summary:
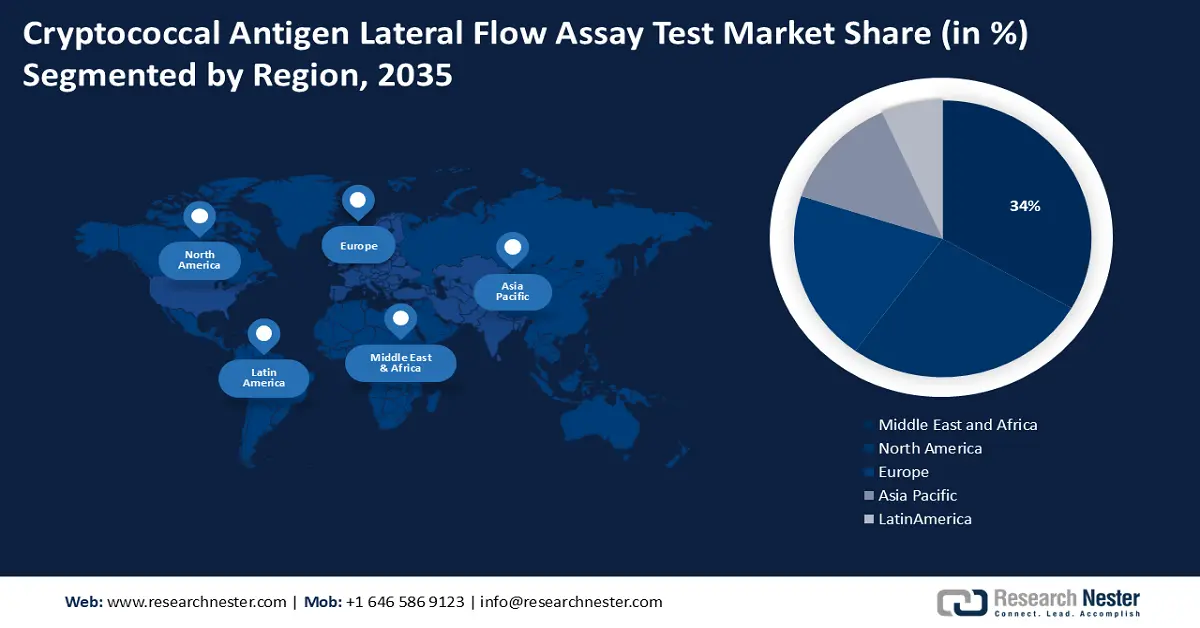
Regional Highlights:
- Across 2026-2035, the cryptococcal antigen lateral flow assay test market in the Middle East and Africa is forecast to command a 34% share by 2035, attributable to intensified healthcare investments addressing high HIV/AIDS prevalence.
- North America is projected to attain a 28% share by 2035, underpinned by expanding R&D efforts and strong adoption of user-friendly lateral flow assays.
Segment Insights:
- By 2035, the serum-based lateral flow assay tests segment in the cryptococcal antigen lateral flow assay test market is expected to account for 56% of total share, fueled by advanced automation and enhanced assay precision.
- The kits and reagents segment is anticipated to secure a 53% share by 2035, supported by increased self-test kit usage and continuous product introductions.
Key Growth Trends:
- Increasing Global Prevalence of HIV/AIDS
- Growing Advancements in Lateral Flow Assay Tests
Major Challenges:
- Limited Awareness Among Individuals
- High Costs Associated with Lateral Flow Assay Tests may Hamper the Market Growth
Key Players: Kestrel Biosciences LIC, Alere Inc., BioMerieux, Danaher Corporation, Forsite Diagnostics, Merck Millipore, Qiagen, Roche Diagnostics, Hologic (Gen-Probe), Siemens Healthcare.
Global Cryptococcal Antigen Lateral Flow Assay Test Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 785.21 million
- 2026 Market Size: USD 812.77 million
- Projected Market Size: USD 1.15 billion by 2035
- Growth Forecasts: 3.9%
Key Regional Dynamics:
- Largest Region: Middle East and Africa (34% Share by 2035)
- Fastest Growing Region: North America
- Dominating Countries: United States, China, Germany, United Kingdom, Japan
- Emerging Countries: India, Brazil, South Korea, Mexico, Indonesia
Last updated on : 28 November, 2025
Cryptococcal Antigen Lateral Flow Assay Test Market - Growth Drivers and Challenges
Growth Drivers
- Increasing Global Prevalence of HIV/AIDS-HIV/AIDS weakens the immune system, increasing a person's vulnerability to opportunistic infections such as meningitis caused by cryptococcal bacteria. According to recent data, in 2022, 39 million people worldwide—between 33.1 million and 45.7 million—were HIV positive. Around 86% of all HIV-positive individuals knew their status and 630 000 persons lost their lives to AIDS-related diseases. The prevalence of cryptococcal infections rises along with the HIV/AIDS epidemic, which fuels demand for diagnostic tests such as the cryptococcal antigen lateral flow assay. Additionally, cryptococcal antigen testing is frequently included in HIV care plans, particularly in areas where the virus is very prevalent. Regular testing for cryptococcal antigenemia in HIV-positive people helps identify those who are susceptible to cryptococcal meningitis, enabling preventive care and treatment.
- Growing Advancements in Lateral Flow Assay Tests- Lateral flow tests with improved sensitivity and specificity have been made possible by improvements in assay design, which include the use of innovative antibody detection techniques. Additionally, several lateral flow assays now include multiplexing, which is the capacity to detect many analytes at once. When it comes to cryptococcal antigen lateral flow tests, multiplexing may make it possible to simultaneously detect additional pertinent indicators, such as HIV antibodies, which would increase the assays' usefulness in a clinical environment. Furthermore, digitizing lateral flow assays lowers user variability, improves test result accuracy and reproducibility, and makes data administration and monitoring easier. Consequently, the cryptococcal antigen lateral flow assay test market is expanding due to a greater integration of digital technology with lateral flow assay testing.
- Enhancing Healthcare Infrastructure in Developing Regions - As healthcare infrastructure becomes more organized and integrated, there is greater emphasis on the implementation of standardized diagnostic protocols and guidelines. Cryptococcal antigen lateral flow assays, known for their rapid and reliable results, can be integrated into these protocols for the diagnosis and management of cryptococcal infections. Therefore, the growing investments in healthcare infrastructure in developing nations are propelling the growth of the market. For instance, under the Coalition for Healthcare Infrastructure in Africa, USTDA programming will involve partnership-building and project preparation to establish technical specifications and draw funding for high-priority healthcare infrastructure projects throughout the continent.
Challenges
- Limited Awareness Among Individuals - Low demand for testing could be caused by patients' lack of knowledge about cryptococcal infection and the accessibility of diagnostic diagnostics. If patients are not aware of the danger of the infection or the necessity of receiving timely diagnosis and treatment, they may choose not to seek medical attention for symptoms suggestive of cryptococcal meningitis. As a result, this factor might prevent the cryptococcal antigen lateral flow assay test market from expanding.
- High Costs Associated with Lateral Flow Assay Tests may Hamper the Market Growth
- Unavailability of Advanced Healthcare Infrastructure in Developing Nations may Hinder Market Growth
Cryptococcal Antigen Lateral Flow Assay Test Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
3.9% |
|
Base Year Market Size (2025) |
USD 785.21 million |
|
Forecast Year Market Size (2035) |
USD 1.15 billion |
|
Regional Scope |
|
Cryptococcal Antigen Lateral Flow Assay Test Market Segmentation:
Type Segment Analysis
Serum-based lateral flow assay tests segment for cryptococcal antigen lateral flow assay test market is expected to hold the largest share of 56% during the foreseen period. Serum-based assays identify particular markers or analytes in the bloodstream, providing useful diagnostic information. Numerous illnesses, such as cancer, autoimmune diseases, hormone abnormalities, and viral infections, can be diagnosed with these tests. Additionally, even at low concentrations, these assays are capable of precisely identifying and measuring target analytes in serum samples. Furthermore, the performance, repeatability, and throughput of serum-based assays have all increased with automation and standardization. To improve laboratory productivity and efficiency, healthcare facilities are progressively implementing automated methods for serum-based testing. Additionally, this test is essential to customized therapy because it helps determine the best course of action for each patient.
Instruments Segment Analysis
The kits and reagents segment for the cryptococcal antigen lateral flow assay test market is anticipated to hold the second-largest share of 53% during the projected period. With the use of these lateral flow kits, users may be more flexible in capturing and identifying any kind of analyte without having to apply the captured antibodies onto the test strip. These kits are utilized in medicines, animal health, environmental testing, feed and food testing, and crop and plant testing because of their versatility. During the projected period, new kit launches by leading manufacturers are expected to stimulate market growth. Also, the growing adoption of self-test kits for COVID-19 and other diseases is accelerating the growth of the segment. For instance, the Kenyan government has introduced two novel technologies, pre-exposure prophylaxis (PrEP) to prevent HIV infection and self-testing for HIV, in the hopes of taking the fight against the AIDS epidemic one step closer. HIV self-test kits are being provided by the government as part of the campaign at a low cost of about USD 8 per kit, which was negotiated in collaboration with the commercial sector and public and private health facilities.
Our in-depth analysis of the global market includes the following segments:
|
Type |
|
|
Instruments |
|
|
Location |
|
|
End-user |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Cryptococcal Antigen Lateral Flow Assay Test Market - Regional Analysis
Middle East and Africa Market Insights
Cryptococcal antigen lateral flow assay test market in the Middle East and Africa industry is predicted to dominate majority revenue share of 34% by 2035. The high prevalence of HIV/AIDS in the region has led to an increase in the incidence of cryptococcal infections, which in turn has increased demand for diagnostic tests. Inadequate laboratory facilities and a dearth of healthcare workers are two issues that many nations in the region confront as a result of their weak healthcare infrastructure. International organizations are consequently making significant investments in these regions' healthcare infrastructure, which is propelling the market's expansion.
North American Market Insights
North American cryptococcal antigen lateral flow assay test market is poised to grow majorly with a share of 28% by the end of 2035. The region boasts a firmly established healthcare network, complete with clinical laboratories outfitted with diagnostic testing capabilities. Furthermore, biotechnology companies actively create novel diagnostic tools, and the regional government makes significant investments in research and development. For instance, the amount invested in medical and health research and development (R&D) in the United States (U.S.) in 2020 was USD 245.1 billion, an increase of 11.1% over 2019. Additionally, the market expansion in this region is being supported by the rising need for lateral flow assays that are simple to use in a variety of healthcare settings.
Cryptococcal Antigen Lateral Flow Assay Test Market Players:
- Kestrel Biosciences LIC
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Alere Inc.
- BioMerieux
- Danaher Corporation
- Forsite Diagnostics
- Merck Millipore
- Qiagen
- Roche Diagnostics
- Hologic (Gen-Probe)
- Siemens Healthcare
Recent Developments
- April 2021 - For the past ten years, the industry-leading Cryptococcal Antigen (CrAg) Lateral Flow Assay (LFA) from IMMY has helped doctors diagnose cryptococcal infections all over the world. Immunocompromised individuals, such as those infected with HIV, are susceptible to cryptococcal infections, and early identification can greatly enhance therapeutic results. Before the onset of cryptococcal meningitis, the CrAg® LFA can identify cryptococcal antigens in the blood of asymptomatic patients, allowing for the proactive treatment of CrAg-positive individuals. This year commemorates the tenth anniversary of this potentially life-saving test, which, when compared to culture or India ink microscopy, has the advantages of rapid turnaround and sensitivity, revolutionizing the detection of an opportunistic fungal infection.
- January 2024 - In October 2023, bioMérieux inked a new Research Master Agreement with the University of Pittsburgh (Pennsylvania, USA), which was in line with its business growth and regional expansion. This three-year contract presents an excellent chance to lead the way in the creation of novel diagnostic approaches in the infectious disease space. Pitt's Division of Clinical Microbiology and Division of Infectious Diseases is working with the bioMérieux Analytical and Sequencing team on this first collaborative project, which has a sequencing focus.
- Report ID: 5845
- Published Date: Nov 28, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Cryptococcal Antigen Lateral Flow Assay Test Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




