CAR T-Cell Therapy Market - Regional Analysis
North America Market Insights
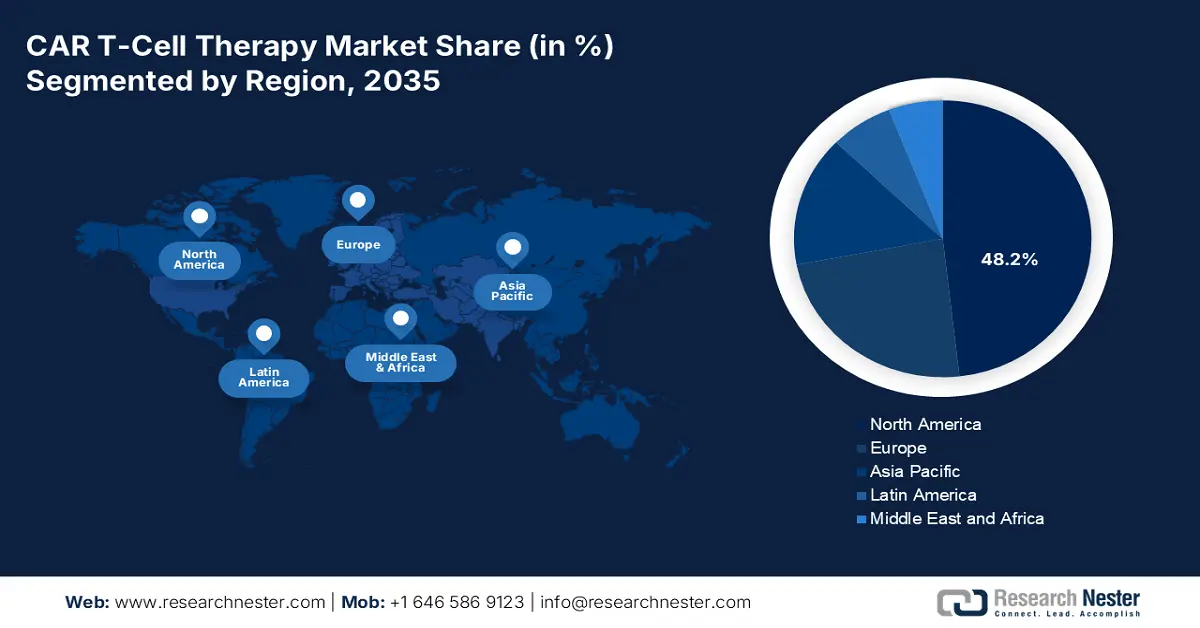
North America is expected to dominate the global market with a share of 48.2% by the end of 2037. The region has a rising patient pool, which is the primary growth driver in the region. Regarding the same, the NLM article published in June 2025 states that patients aged 60+ received axicabtagene ciloleucel (Yescarta) with effective responses and manageable side effects. Further, the established network of advanced cancer care centers across developed countries, such as the U.S. and Canada, also contributes to a greater rate of adoption in this sector.
The U.S. is augmenting the regional market with dominance on account of strong insurer backing and substantial Federal investments. The CDC report released in June 2025 declares that in 2022, nearly 1,851,238 new cancer cases were reported, urging for CAR T cell therapy in the U.S. On the other hand, the government is increasing the funds for cancer research, including CAR T therapies. Medicare and Medicaid have expanded reimbursement policies to cover the patient population and enhance therapy accessibility. Clinical trials, patient access, and manufacturing capacity also drive the market growth.
Cancer Burden Statistics in U.S. and Canada
|
Country |
Year |
New Cases |
Deaths |
|
U.S. |
2023 |
1,958,310 |
609,820 |
|
Canada |
2023 |
239,100 |
86,700 |
|
U.S. |
2024 |
2,001,140 |
611,720 |
|
Canada |
2024 |
247,100 |
88,100 |
Source: Cancer Progress Report, Canadian Cancer Statistics, Canadian Cancer Society
APAC Market Insights
Asia Pacific is poised to register the fastest pace of growth in the global CAR T-cell therapy market by the end of 2037. Countries like China, Japan, Korea, and Singapore have performed various CAR T-cell clinical trials, supported by government and philanthropic funding. For example, China initiated over 342 clinical trials by 2021, as reported in NLM study in December 2023. In addition, the increasing occurrence and mortality of chronic disorders, such as cancers, cardiovascular disease, and diabetes, are presenting a broad range of applications for this merchandise. As a result, both domestic and foreign companies are becoming keener to invest and participate in this landscape to captivate a greater proportion of profit margins.
The emergence of India as a key biopharmaceutical therapeutic developer is solidifying its lucrative augmentation in the CAR T-cell therapy market. India's first homegrown CAR-T cell therapy, NexCAR19, shows a 73% success rate against cancer, according to the India Today article published in March 2025. Further, the NexCAR19 is expected to cost $50,000 and is projected to treat 1,200 patients in a year, based on the National Cancer Institute report in February 2024. The government supports its integration into healthcare via policy initiatives and infrastructure development, making India a growing hub for advanced cancer immunotherapy.
Europe Market Insights
The CAR T-cell therapy market in Europe is estimated to hold a notable share by 2035. This is accomplished through comprehensive pricing, accelerated EMA compliance, and increasing hematologic cancer cases. Over 30% of approved advanced therapy medicinal products in the UK/EU are CAR-T therapies; 63% target blood cancers, 37% solid tumors, mainly gastrointestinal, breast, and nervous system, based on the Science Direct report released in November 2024. On the other hand, the strengthening emphasis on cost-effective innovation and standardized access is making this region a major consumer base and policy leader in advanced CAR-T cell therapies globally.
The UK dominates the regional CAR T-cell therapy market with provincial government funding and the presence of international biotechnological pioneers. The nation is propelled by increasing hematologic cancer incidences, growing R&D spending, and favorable regulatory environments. According to the Macmillon Cancer Support article posted in January 2021, in the UK, CAR-T therapy treats selected B-cell leukemias and lymphomas, reaching around 200 patients each year. NHS programs and government funds are also driving market growth, whereas partnerships among industry and academia increase innovation and clinical trial activity, making the nation a leader in CAR T-cell therapy in Europe.