Candidiasis Drugs Market - Regional Analysis
North America Market Insights
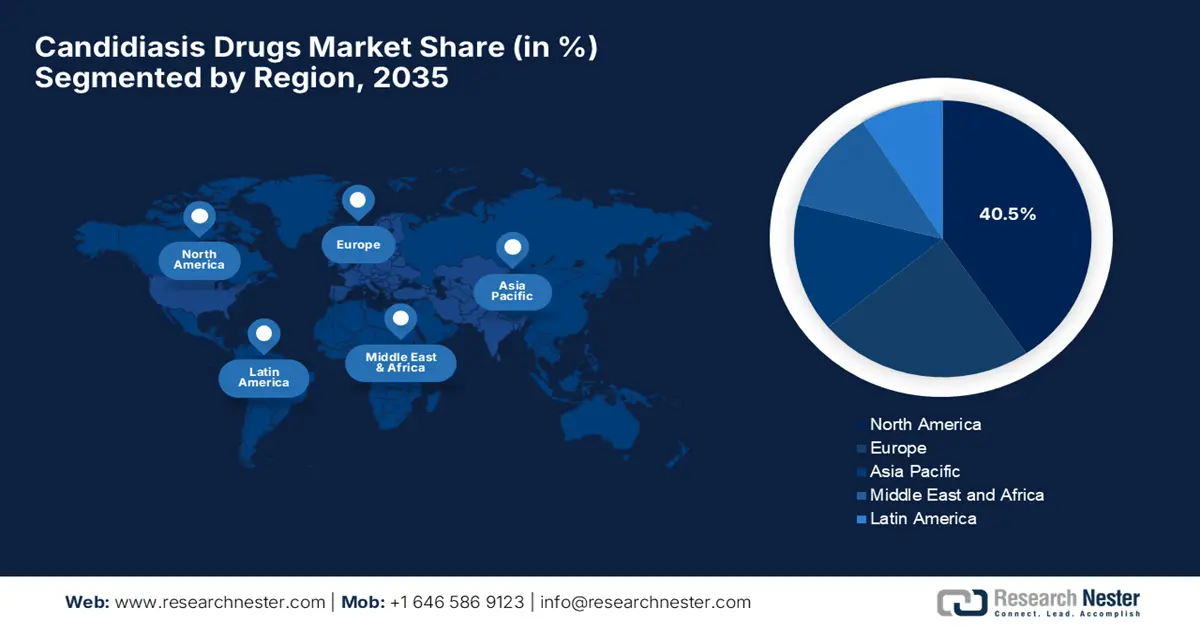
North America is expected to capture the largest share of 40.5% in the candidiasis drugs market by the end of the analyzed timeline. This leadership is pledged to its advanced healthcare infrastructure and upgradation in product formulations. In March 2023, GSK and SCYNEXIS together reported that they have entered an alliance to commercialize and further develop Brexafemme (ibrexafungerp), which is the first U.S. FDA-approved oral antifungal for vulvovaginal candidiasis and recurrent VVC. The company also stated that SCYNEXIS will gain USD 90 million upfront, while retaining rights to other enfumafungin-derived assets.
The U.S. is augmenting its dominance over the regional candidiasis drugs market, backed by substantial federal investments and healthcare escalations. Besides, the implementation of AI diagnostics reduced hospitalization due to related infections, fueling a surge in non-invasive treatment options. As evidence, NIH in July 2025 revealed that an estimated 133,555 fungal disease-related hospitalizations and 13.37 million outpatient visits occur on a yearly basis in the U.S., leading to USD 13.4 billion in direct medical costs. Out of these, invasive candidiasis caused the most hospitalizations, i.e., 61,120, and USD 3.3 billion in costs.
The Canada candidiasis drugs market is also experiencing significant growth on account of strong and ambitious government commitments toward healthcare advancements. For instance, in January 2024, Medexus Pharmaceuticals stated that its new drug submission for a topical terbinafine hydrochloride nail lacquer, for treating fungal nail infections, has been accepted for review by Health Canada, which marks a major milestone towards the treatment of fungal infections in the country.
Direct Medical Costs of Candidiasis in the U.S. by Payer Type (2024 USD)
|
Type |
Medicaid |
Medicare |
Private Insurance |
Other |
Total |
|
Hospitalization -Invasive |
421,712,709 |
1,707,767,031 |
1,904,536,553 |
199,631,338 |
4,233,647,630 |
|
Hospitalization Non-invasive/Unspecified |
12,375,103 |
44,612,841 |
54,613,536 |
4,509,375 |
116,110,854 |
|
Outpatient - Invasive |
14,730,787 |
14,460,998 |
57,736,515 |
8,475,085 |
95,403,384 |
|
Outpatient - Non-invasive/Unspecified |
69,147,577 |
260,790,328 |
490,590,734 |
72,013,319 |
892,541,958 |
|
Total Candidiasis (All Types) |
517,966,176 |
2,027,631,198 |
2,507,477,338 |
284,629,117 |
5,337,703,829 |
Source: NIH
APAC Market Insights
Asia Pacific is predicted to register the highest pace of growth in the global candidiasis drugs market between 2026 and 2035. This propagation is highly stimulated by the rising incidences of fungal infections, increasing populations of diabetes, HIV, and cancer, and widespread antibiotic overuse cases. China and India are taking leadership in this patient pool. Furthermore, the presence of technologically developed countries, such as Japan and South Korea, is focused on AI-based innovations through extensive R&D activities and investments.
China is marking its regional dominance over the candidiasis drugs market due to having a large patient pool and exceptional API production capacity. In February 2024, Mycovia Pharmaceuticals announced the launch of VIVJOA (oteseconazole) capsules by their partner Jiangsu Hengrui Pharmaceuticals in the country for treating severe vulvovaginal candidiasis. The company further stated that the innovative oral azole antifungal offers a 2-day treatment regimen, enabling a strong capital influx, benefiting this sector.
India is also following the accelerated progress of the Asia Pacific candidiasis drugs market, which is backed by its biologics production capacity, government efforts to bridge the access gap in rural hospitals, and an enlarging patient population. In December 2023, Aurobindo Pharma, also known as Eugia Pharma Specialities Limited, received final approval from the U.S. FDA for the manufacturing and marketing of Posaconazole Injection, a medication designed to help prevent invasive Aspergillus and Candida infections in immunocompromised patients.
Europe Market Insights
The candidiasis drugs market in Europe is portraying a steady growth facilitated by increasing awareness about fungal infections and the rising prevalence of immunocompromised patients. Besides advancements in antifungal therapies, along with improved diagnostic techniques, there are also providing greater opportunities for players to gain the interest of both service providers and consumers. In December 2023, Mundipharma announced that the European Commission had approved REZZAYO (rezafungin acetate) for the treatment of invasive candidiasis in adults and is the best alternative for seriously ill patients with high mortality rates.
Germany has a huge potential to capitalize on the candidiasis drugs market, effectively driven by a strong focus on research and development, supported by a robust healthcare system. The country’s market also benefits from a huge emphasis on early diagnosis and personalized treatment approaches, thereby driving demand for effective antifungal therapies. Furthermore, the country’s well-established pharmaceutical sector and amplifying collaborations between public and private entities are propelling greater progress in this field.
The U.K. also holds a strong position in the candidiasis drugs market due to the increasing instances of fungal infections and the pivotal role of the National Health Service (NHS) in ensuring access to antifungal medications. For example, in September 2025, researchers led by Professor Miraz Rahman at King’s College London received a £1.45 million (USD 1.7 million) grant to develop new antifungal drugs targeting drug-resistant Candida auris and other Candida species, thereby aiming to create efflux-resistant antifungal agents.