Allergic Conjunctivitis Market Outlook:
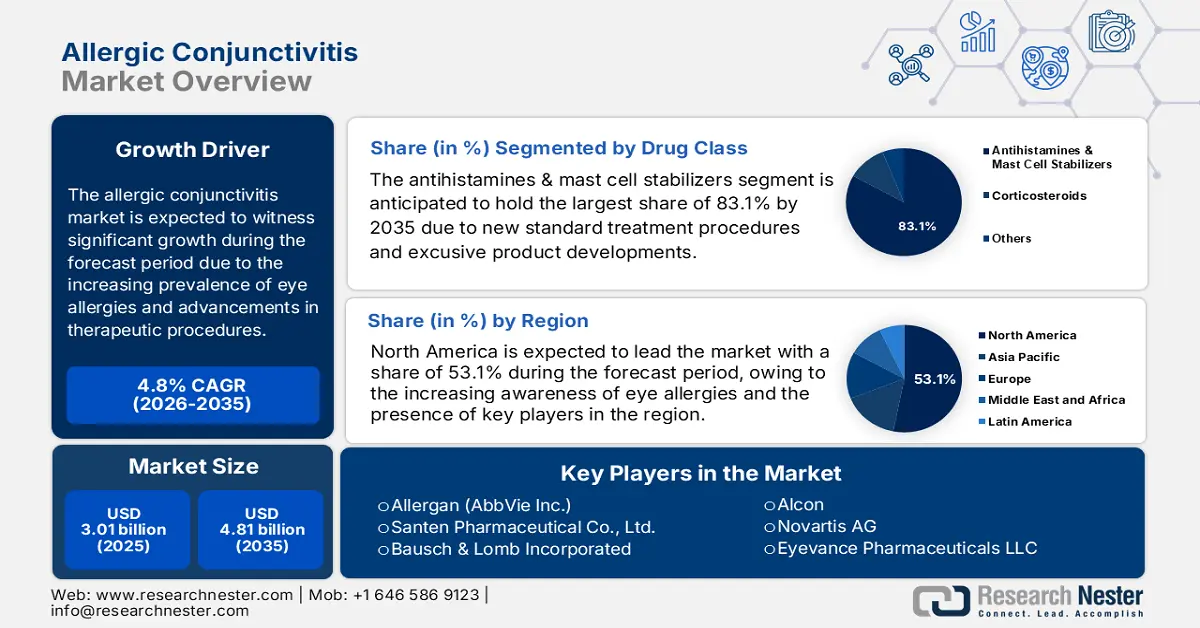
Allergic Conjunctivitis Market size was valued at USD 3.01 billion in 2025 and is set to exceed USD 4.81 billion by 2035, registering over 4.8% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of allergic conjunctivitis is estimated at USD 3.14 billion.
The allergic conjunctivitis market is experiencing steady growth with immense support from increasing instances of allergic conjunctivitis across the world and the rising awareness among both patients and healthcare professionals to avail effective therapeutic measures. Rapid urbanization and a surge in the number of allergens are further contributing to the market expansion. According to the NLM report published in January 2024, the condition affects 40% of the population globally; it further reported that 10% to 30% of the general population are being diagnosed with allergic conjunctivitis in a year, with people aged below 20 being the most affected. This denotes the need for worthwhile medical therapies supporting market upliftment.
Furthermore, the increasing healthcare expenditure and the adoption of innovative drug formulations drive business in the industry. The augmented use of therapeutic drugs and ophthalmic solutions requires proactive regulatory approvals to help mitigate risks, build trust, and accelerate market growth. For instance, in February 2021, Bausch + Lomb Corporation launched Alaway non-preservative ketotifen fumarate ophthalmic solution 0.035%, a U.S. FDA-cleared antihistamine eye drop, the first and only OTC to relieve itchy eyes. These launch activities, with support from the regulatory framework, are anticipated to boost the market growth further.
Key Allergic Conjunctivitis Market Insights Summary:
Regional Highlights:
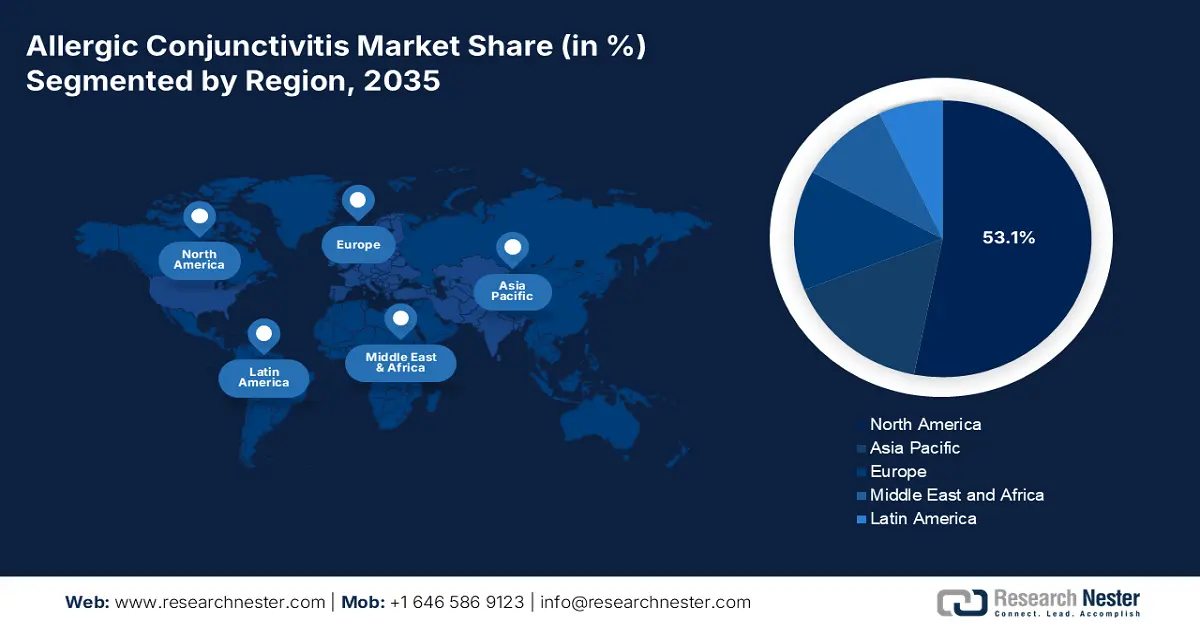
- North America commands the Allergic Conjunctivitis Market with a 53.1% share, driven by high burden and awareness of ocular diseases, pharmaceutical launches, and early adoption of advanced treatments, ensuring robust growth through 2035.
- Asia Pacific’s allergic conjunctivitis market is anticipated to grow rapidly by 2035, driven by increasing ocular allergy incidence, rising medical expenditure, and government-promoted awareness campaigns.
Segment Insights:
- The Antihistamines & Mast Cell Stabilizers segment is anticipated to achieve 83.1% market share by 2035, driven by their effectiveness in alleviating allergic conjunctivitis symptoms.
Key Growth Trends:
- Advancements in treatment measures
- Increasing awareness and diagnosis rates
Major Challenges:
- Limited long-term treatment options
- Underdiagnosis or misdiagnosis
- Key Players: Santen Pharmaceutical Co., Ltd., Bausch & Lomb Incorporated, Alcon, Novartis AG, Eyevance Pharmaceuticals LLC, Sun Pharmaceutical Industries Ltd..
Global Allergic Conjunctivitis Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 3.01 billion
- 2026 Market Size: USD 3.14 billion
- Projected Market Size: USD 4.81 billion by 2035
- Growth Forecasts: 4.8% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (53.1% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, Japan, United Kingdom, France
- Emerging Countries: China, India, Brazil, Russia, Mexico
Last updated on : 12 August, 2025
Allergic Conjunctivitis Market Growth Drivers and Challenges:
Growth Drivers
- Advancements in treatment measures: The rapid development of technologies such as targeted therapies and improved ocular drug delivery systems is a key driver for the allergic conjunctivitis market. Continuous innovation in treatments is inspiring the pharmaceutical companies to invest in novel formulations that offer enhanced efficacy with minimal side effects. In March 2020, Nicox SA announced that its partner firm, Eyevance Pharmaceuticals, launched ZERVIATE in the U.S. for allergic conjunctivitis by enhancing its existing product portfolio. These advancements are making allergic conjunctivitis solutions more reliable, scalable, and accessible across the globe.
- Increasing awareness and diagnosis rates: Another major fueling factor for the market is the growing awareness about eye-associated allergic conditions and the significance of timely interventions. For instance, in October 2023, Okogen Inc. began a Phase IIb clinical trial to analyze OKG-0303 for acute infectious conjunctivitis in India. This enhanced awareness supports the unmet medical needs of the large patient population; further supporting the growth trajectory of the market with healthy competition between the global players.
Challenges
- Limited long-term treatment options: One of the biggest challenges in the global market is the lack of effective long-term treatment options. Most available therapies, such as antihistamines and corticosteroids, provide relief, but at times it might be temporary and are associated with side effects upon long-term usage. Chronic cases require sustained treatment, but the risk of ocular complications such as increased intralocular pressure and cataract formation from prolonged steroid use limits their applicability, hindering market penetration.
- Underdiagnosis or misdiagnosis: Another major challenge in the market is the underdiagnosis and frequent misdiagnosis of the condition, particularly in regions with limited access to specialized eye care. Symptoms such as redness, itching, and tearing are often mistaken for other common eye conditions, leading to inappropriate or delayed treatment. This lack of standardization hinders scalability, limiting the ability of healthcare professionals and patients to fully leverage the benefits of allergic conjunctivitis technologies.
Allergic Conjunctivitis Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
4.8% |
|
Base Year Market Size (2025) |
USD 3.01 billion |
|
Forecast Year Market Size (2035) |
USD 4.81 billion |
|
Regional Scope |
|
Allergic Conjunctivitis Market Segmentation:
Durg(Antihistamines & Mast Cell Stabilizers, Corticosteroids)
Based on drug class, the antihistamines & mast cell stabilizers segment is expected to register the largest share of 83.1% in the allergic conjunctivitis market during the forecast period. The dominance of the segment is attributable to the effectiveness of these elements in alleviating the symptoms associated with allergic conjunctivitis as an ideal therapeutic option. As per a clinical study by NLM in January 2024, mast cell stabilizers such as sodium cromoglicate, lodoxamide, others, and antihistamines, Emedastine, epinastine, others, help reduce inflammation and are effective in rapidly controlling allergic responses in vernal keratoconjunctivitis and atopic keratoconjunctivitis. Thus, this denotes a positive outlook for the segment’s growth.
Distribution Channel (Hospital Pharmacies, Drug Stores, Retail Pharmacies, and Online Pharmacies)
Based on the distribution, the hospital pharmacies segment is projected to register considerable growth in the allergic conjunctivitis market by 2035. The segment’s dominance is attributable to the adoption of these settings for the diagnosis and prescription after a concise examination by the healthcare professional. As per the July 2024 NLM report, ophthalmic solutions play a critical role in seasonal ad perineal allergic conjunctivitis, and antihistamines and dual-action agents are recommended for the treatment. It is intended to support hospital pharmacies with a need for ophthalmic formulations and clinical guidance for safer, smarter, and prescription-based treatments.
Our in-depth analysis of the global market includes the following segments:
|
Drug Class |
|
|
Disease Type |
|
|
Distribution Channel |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Allergic Conjunctivitis Market Regional Analysis:
North America Market Analysis
North America is a key player in the allergic conjunctivitis market, projected to register a significant share of 53.1% in 2035. The dominance of the region is attributable to the increased burden of ocular diseases, their awareness, and the existing pharmaceutical base. This has encouraged the global players to launch more of such formulations. In July 2023, Harrow Inc. acquired the U.S. and Canada commercial rights to Santen’s branded ophthalmic products, including FLAREX, NATACYN, TOBRADEX ST, VERKAZIA, and ZERVIATE. The agreement also covers non-prescription brands FRESHKOTE and Cationorm PLUS. Hence, the move strengthens the company’s presence in the North America eyecare pharmaceutical market.
U.S. dominates the North America market owing to early adoption of cutting-edge treatment technologies. For instance, in February 2020, Alcon launched Pataday Once Daily Relief (olopatadine hydrochloride ophthalmic solution 0.2%) and Pataday Twice Daily Relief (olopatadine hydrochloride ophthalmic solution 0.1%) after receiving the U.S FDA clearance. These launches are to address the concerns of ocular diseases in the region and will amplify the market expansion during the forecast period. Additionally, the strong presence of global pharmaceutical leaders and an innovation-driven economy make the U.S. a hub for conjunctivitis advancements.
The Canada market is growing steadily with several collaborative approaches, including public-private partnerships, ensuring a robust treatment adoption across key sectors. In February 2024, Harrow Inc. and Apotex Inc. entered into an exclusive out-licensing agreement granting Apotex rights to market and distribute VERKAZIA for vernal keratoconjunctivitis, Cationorm PLUS to relieve dryness and irritation. Seek approval in Canada for VEVYE for dry eye disease, IHEEZO, and ZERVIATE for ocular itching due to allergic conjunctivitis. The deal aims to expand access to key ophthalmic treatments in the Canada market by leveraging their ecosystems and positioning Canada for global markets.
APAC Market Statistics
The Asia Pacific allergic conjunctivitis market is anticipated to grow at the fastest rate during the forecast period, driven by increasing instances of ocular allergies in the region. Governments in the region actively promote smart health campaigns, due to which the awareness increases, allowing substantial medical expenditure in the Asia Pacific. Moreover, countries such as China, India, and Japan are witnessing growing demand for eye care solutions as exposure to environmental pollutants and allergens intensifies densely populated areas placing Asia Pacific as key contributor for market progression.
China is a leader in the market, which is exceptionally supported by the increasing awareness of availing effective treatment for the disease. Moreover, the country’s supportive government, which emphasizes innovative management modalities, has fostered a favorable business environment for this sector. In September 2024, Nicox SA declared that its China-based partner, Ocumension Therapeutics, received approval for commercializing ZERVIATE (cetirizine ophthalmic solution) in China at a 0.24% concentration, for ocular itching, which is a symptom of allergic conjunctivitis. With these approvals, the country’s market continues to expand rapidly, shaping the region’s healthcare landscape.
The India market is gaining traction, fueled by the rising medical expenditure for eye care and the availability of innovative therapeutic regimens. The evolution of ophthalmic suspensions, antihistamines, and mast cell stabilizers is further accelerating growth in the region. For instance, in April 2024, Lupin Limited notified that it received the U.S FDA clearance for Loteprednol Etabonate, an ophthalmic suspension, 0.5%, to alleviate the symptoms of seasonal allergic conjunctivitis. Additionally, the push for accessible eye care is prompting firms to adopt allergic conjunctivitis therapies, making India a rapidly emerging player in the global market.
Key Allergic Conjunctivitis Market Players:
- Allergan (AbbVie Inc.)
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Santen Pharmaceutical Co., Ltd.
- Bausch & Lomb Incorporated
- Alcon
- Novartis AG
- Eyevance Pharmaceuticals LLC
- Sun Pharmaceutical Industries Ltd.
- Ocular Therapeutix, Inc.
- Eton Pharmaceutical
- ALK-Abelló AS
- Aldeyra Therapeutics, Inc
- Okogen Inc.
- Nicox SA
- Harrow Inc.
- Ocumension Therapeutics
- Lupin Limited
Companies involved in the allergic conjunctivitis market are emphasizing acquisition strategies to enhance their pharmaceutical portfolio and improve patient outcomes in ocular surface diseases. Moreover, they focus on exclusive product launches, and geographical expansion to accelerate adoption and expand market presence. For instance, in June 2023, Bausch + Lomb Corporation acquired XIIDRA (lifitegrast ophthalmic solution) 5%, a non-steroidal eye drops from Novartis AG to treat inflammation associated with dry eye. Thus, these acquisitions will enhance their existing product portfolio thereby widening market scope.
Some of the prominent market players are:
Recent Developments
- In April 2025, Aldeyra Therapeutics, Inc., notified that it received a Complete Response Letter from the U.S. FDA for the resubmission of reproxalap, for dry eye disease, which is a new drug application.
- In December 2024, Okogen Inc. finalized a financing of USD 3.3 million, aiming to advance the phase 2b clinical program for acute infectious conjunctivitis and develop an AI-based & picture-based platform, which is designed for telehealth and home-based application.
- Report ID: 7666
- Published Date: Aug 12, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Allergic Conjunctivitis Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




