Acute Hepatic Porphyria Treatment Market Outlook:
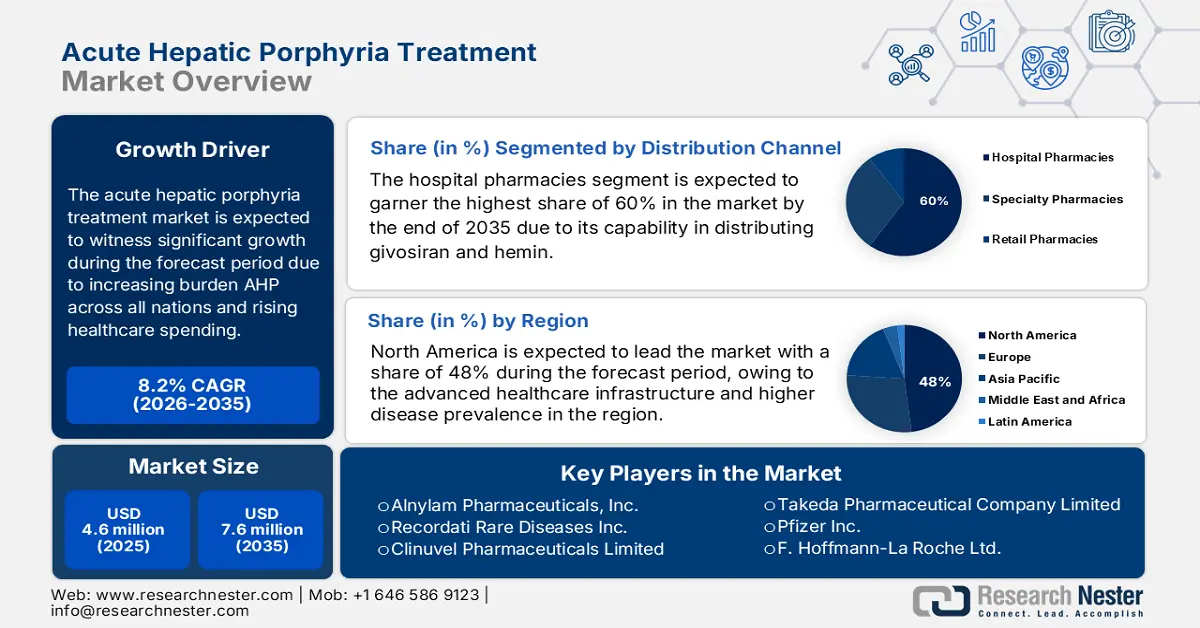
Acute Hepatic Porphyria Treatment Market size was estimated at USD 4.6 million in 2025 and is projected to reach USD 7.6 million by the end of 2035, rising at a CAGR of 8.2% during the forecast period, i.e., 2026-2035. In 2026, the industry size of acute hepatic porphyria treatment is assessed at USD 920.6 million.
The global acute hepatic porphyria treatment market is evolving rapidly, driven by its extensive patient pool and ongoing technological advancements in therapeutic procedures. The NLM study in December 2024 has clearly depicted that the symptomatic population prevalence ratio is 1:20,000. The supply chain for AHP treatments is highly specialized, involving the synthesis of complex active pharmaceutical ingredients (APIs) like hemin and givosiran under stringent current good manufacturing practices. Further, rising government and institutional investments in rare disease research are supporting the development and wider availability of acute hepatic porphyria therapies worldwide.
Additionally, the global trade in AHP treatments is witnessing significant developments. In 2023, the OEC states that Germany is marked as a major exporter of pharmaceutical products, accounting for USD 115 billion, and similarly, the U.S. is the leading importer, holding the worth of USD 170 billion. Besides, the research activities also drive business in the sector, gaining the interest of global leaders to invest in such therapeutics. In this regard, in March 2025, the Ministry of Health and Family Welfare has allocated Rs. 118.82 crore for R&D in rare diseases for the fiscal year 2024-2025. Similarly, Europe gained public-private funding initiatives to focus on gene therapy and heme biosynthesis modulation. Hence, these factors will readily boost the acute hepatic porphyria treatment market development by 2035.