Womens Health Diagnostics Market - Regional Analysis
North America Market Insights
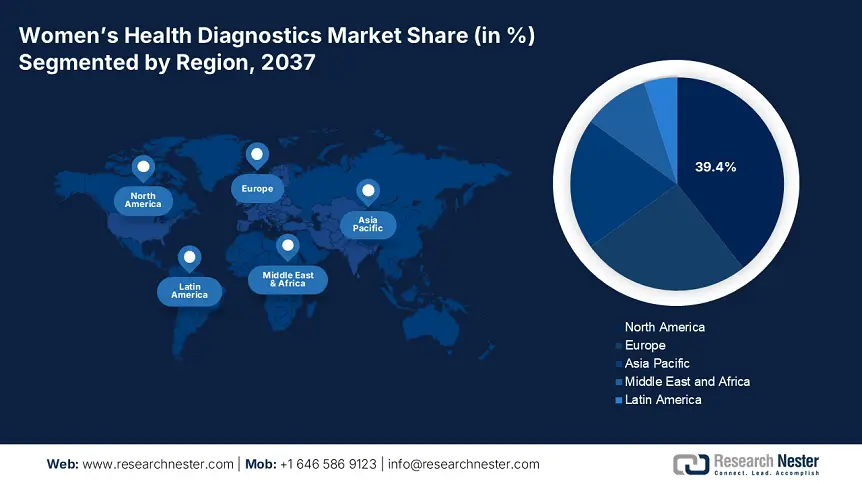
The North America market is projected to account for a leading share of 39.4% by the end of 2037. The growth is led by the early screening programs, government funding, and support from Medicaid and Medicare. In 2024, the CMS boosted Medicaid coverage for diagnostics by over 6%, setting aside around USD 1 billion for related treatments.
Over in Canada, Health Canada and CIHI noted over 7% federal healthcare allocation for this area in 2023. These financial commitments are enhancing access to mammograms, Pap smears, and genetic screenings. This, in turn, is leading to higher diagnosis rates and earlier interventions. Canada’s womens health diagnostics market is thriving. Provinces like Ontario and British Columbia have made significant improvements to their diagnostic infrastructure. Moreover, BioteCanada and Innovative Medicines Canada are supporting research and development partnerships with public hospitals to test next-generation genetic diagnostics.
Europe Market Insights
Europe is poised to hold a revenue share of 25% throughout the forecast period due to the growing awareness of public health, national screening programs, and funding initiatives. There's a strong focus on the early detection of breast, cervical, and ovarian cancers, which has prompted policy and budget alignment among member states. With aging populations, rising rates of female-specific health issues, and a commitment to health equity, countries are ramping up their investments.
Germany is at the forefront of the womens health diagnostics market in Europe. This impressive growth can be attributed to the increased availability of reimbursement options for digital diagnostics. Germany has launched a federal initiative focused on early diagnosis for underserved migrant and elderly communities. France’s womens health diagnostics market is set to represent over 17% of the European market by 2037. A significant development is the introduction of performance-based reimbursements for regional hospitals that offer women-specific diagnostics. Digital screening tools approved by HAS are currently being tested in regional health centers.