Triamcinolone Market Outlook:
Triamcinolone Market size is valued at USD 944.2 million in 2025 and is projected to reach USD 1,332.3 million by the end of 2035, rising at a CAGR of approximately 3.9% during the forecast period, i.e., 2026-2035. In 2026, the industry size of triamcinolone is estimated at USD 981 billion.

The worldwide market is growing due to increasing demand for corticosteroid treatment in respiratory, osteoarthritis, multiple sclerosis, and dermatology applications. Growth is supported by rising cases of chronic allergic conditions and inflammatory skin disorders, along with better access to healthcare and manufacturing in countries with specialized API and dosage production. The global supply system relies on centralized manufacturing of active pharmaceutical ingredients (APIs), mainly in India and China, with formulation and packaging mostly done in North America and Europe.
Global trade flows of the market are strong indicators of production and distribution capacity in some pharmaceutical sectors. According to a report by NIH, May 2023, corticosteroids are among the most widely prescribed drug classes worldwide, with an estimated annual market of over USD 10 billion. These volumes indicate robust manufacturing capacity and consistent demand in countries for corticosteroid-based therapy. Regulatory approval for new formulations and newer delivery systems, including injectables and topical sprays, is expanding the therapeutic indications of triamcinolone. Moreover, the availability of inexpensive generic forms of triamcinolone is driving wider use, especially in emerging economies.
Key Triamcinolone Market Insights Summary:
Regional Insights:
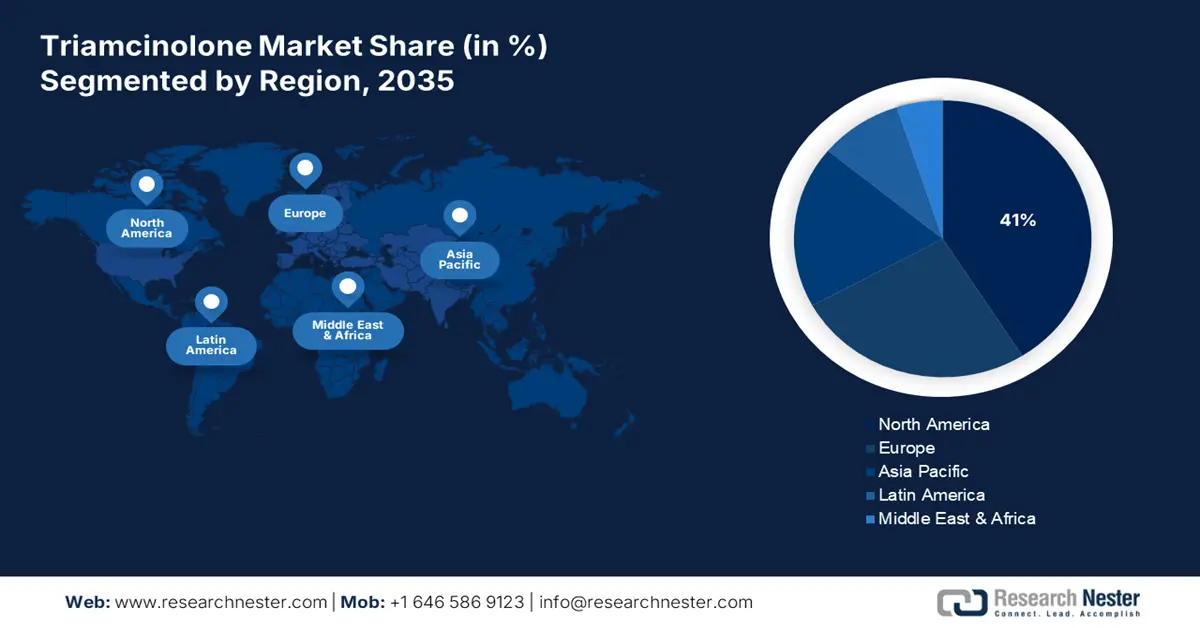
- The North America region is projected to hold a 41% share by 2035 in the Triamcinolone Market, owing to the high prevalence of chronic inflammatory and dermatological disorders and strong pharmaceutical R&D infrastructure.
- The Asia Pacific region is expected to witness the fastest growth during 2026–2035, impelled by increased healthcare expenditure, expanding patient base for dermatological conditions, and growing penetration of generic and OTC corticosteroid treatments.
Segment Insights:
- The hospitals sub-segment in the end user category is projected to account for 51% share by 2035 in the Triamcinolone Market, propelled by the increasing incidence of inpatient and outpatient visits for skin, respiratory, and joint-related illnesses.
- The topical sub-segment in the route of administration category is anticipated to capture the largest share by 2035, driven by ease of use, targeted delivery, and fewer systemic side effects.
Key Growth Trends:
- Rising incidence of corticosteroid-induced bone complications
- Proven efficacy coupled with safety concerns
Major Challenges:
- Safety concerns and side effects limiting usage
- Supply chain and regulatory complexities
Key Players: Johnson & Johnson, Teva Pharmaceutical Industries, Mylan (Viatris), Sanofi, Bristol-Myers Squibb, Novartis, GlaxoSmithKline (GSK), Merck & Co., Pfizer, Sun Pharmaceutical Industries, Dr. Reddy's Laboratories, Lupin Limited, Zydus Cadila, Almirall, Bayer.
Global Triamcinolone Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 944.2 million
- 2026 Market Size: USD 981 billion
- Projected Market Size: USD 1332.3 million by 2035
- Growth Forecasts: 3.9% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (41% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: China, United States, Japan, Germany, South Korea
- Emerging Countries: India, Canada, Indonesia, Australia, Mexico
Last updated on : 30 September, 2025
Triamcinolone Market - Growth Drivers and Challenges
Growth Drivers
- Rising incidence of corticosteroid-induced bone complications: Incidents of corticosteroid-related bone disorders such as osteoporosis and fractures have been rising, increasing the need for safer therapies such as triamcinolone with greater selectivity. According to a report by NIH, May 2023, even low-dose corticosteroids can reduce bone mineral density and raise fracture risk within 3 to 6 months, with doses as low as 5 mg/day of prednisolone or its equivalent. Additionally, osteonecrosis affects up to 40% of patients on long-term corticosteroid therapy, highlighting the need to reevaluate and optimize treatment approaches. These concerns are driving the growth of advanced corticosteroid formulations with improved safety profiles in the market.
- Proven efficacy coupled with safety concerns: Triamcinolone acetonide's powerful anti-inflammatory and immunosuppressive effects, mediated via glucocorticoid receptor activity, make it highly suitable for widespread clinical use. However, recent clinical evidence highlights that even low-dose corticosteroids can make a significant impact on bone mineral density loss and fracture risk in a matter of months of treatment. This has aroused interest in maximized corticosteroid therapy, such as triamcinolone acetonide, which is designed for targeted release and less systemic action. Efficacy over safety remains a major growth driver in the expanding market.
- Increasing prevalence of chronic inflammatory and allergic conditions: The Growing incidence of chronic allergic and inflammatory disorders across the world is a major growth driver of the market. With growing incidence due to environmental and lifestyle risk factors for asthma, allergic rhinitis, and skin diseases, demand for an effective corticosteroid therapy increases. The efficacy of triamcinolone in reducing inflammation and symptom management in various patient groups supports its continued uptake and propels market growth across the world.
Global Countries and Healthcare Expenditure (2022 and 2023)
Healthcare Expenditure of Countries (% of GDP) (2022 and 2023)
|
Country |
Region |
Most Recent Year |
Healthcare Expenditure (%) |
|
U.S. |
North America |
2022 |
16.5 |
|
Germany |
Europe |
2023 |
11.8 |
|
Japan |
Asia |
2022 |
11.4 |
|
U.K |
Europe |
2023 |
10.8 |
|
China |
Asia |
2022 |
5.3 |
|
Brazil |
Latin America |
2022 |
9.1 |
|
South Africa |
Africa |
2022 |
8.7 |
|
Australia |
Oceania |
2022 |
9.9 |
Source: World Bank, 2025
Challenges
- Safety concerns and side effects limiting usage: In the treatment of various disorders, this triamcinolone drug has proved very effective in the market. However, its use is often limited due to safety concerns related to corticosteroid side effects, such as osteoporosis, skin thinning, and adrenal suppression. High-dose therapy or prolonged treatment poses a rather high risk of serious complications, including fractures and osteonecrosis. Such doses and evaluation steps may diminish patient compliance and prescribers' willingness. Demand for alternative therapies with fewer side effects is encouraged by these safety challenges, which in turn hinder the market growth.
- Supply chain and regulatory complexities: The market is facing challenges connected to complex supply chains that involve sourcing raw materials, API manufacturing, and formulation, often carried out in multiple countries. These issues increase production costs and delay the market entrance, arising from regulatory bottlenecks such as stringent quality control, compliance with varying standards across countries, and lengthy approval processes. These factors hamper manufacturers in timely product availability, increasing operational expenses, and thus curbing swift market expansion and competitiveness.
Triamcinolone Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
3.9% |
|
Base Year Market Size (2025) |
USD 944.2 million |
|
Forecast Year Market Size (2035) |
USD 1,332.3 million |
|
Regional Scope |
|
Triamcinolone Market Segmentation:
End user Segment Analysis
The hospitals sub-segment in the end user segment is expected to hold the highest market share of 51% within the forecast period due to the increasing incidence of inpatient and outpatient visits for skin, respiratory, and joint-related illnesses. As per a report by the American Hospital Association, January 2025, there are presently 5,112 community hospitals in the U.S., with 2,978 being not-for-profit community hospitals, 1,214 investor-owned (for-profit) hospitals, and 920 state and local government hospitals, each of which contributes to the delivery of corticosteroid therapy. Additionally, better reimbursement structures and a centralized procurement system make hospitals preferred channels for the prescription and administration of triamcinolone.
Route of Administration Segment Analysis
The topical sub-segment in the route of administration segment is expected to hold the highest market share within the forecast period due to ease of use, targeted delivery, and fewer systemic side effects. Topical formulations are different for the treatment of localized conditions, making these preferred by both patients and providers. With advancements in topical corticosteroid formulations and rising awareness about skin health, this segment is seeing upward growth. Self-administration and better patient compliance further raise the adoption of topical triamcinolone products.
Application Segment Analysis
The dermatology sub-segment in the application segment is expected to hold the highest market share within the forecast period due to the widespread prevalence of chronic skin conditions such as eczema, psoriasis, and dermatitis. Triamcinolone is one of the most important therapeutic agents available to reduce inflammation and treat symptoms. Growing awareness amongst patients and the desire for effective skin care treatments also fuel the dominance of the dermatological application segment. Moreover, the advancement of research and product development continues to increase the range of dermatological applications of triamcinolone.
Our in-depth analysis of the global market includes the following segments:
|
Segment |
Sub-segments |
|
Type |
|
|
Formulation |
|
|
Application |
|
|
Route of Administration |
|
|
End user |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Triamcinolone Market - Regional Analysis
North America Market Insights
The triamcinolone market in North America is expected to hold the highest growth rate with a 41% market share within the forecast period due to the presence of ample patients with chronic inflammatory and dermatological disorders, well-established pharmaceutical R&D infrastructure, and robust drug distribution networks. The government policies promoting access to more affordable drugs, for example, fast-track approvals for generics, are supporting the growth. Investment in committed R&D by leading pharmaceutical companies is driving innovation in novel delivery forms such as injectables and inhalables. Company regulatory steps provide assurances of drug safety without ruling out the timely availability of new therapies to the market.
The triamcinolone market in the U.S. is expected to grow steadily due to its advanced healthcare systems with easy patient availability for dermatology care, good reimbursements for corticosteroids, and an understanding period when skin diseases are actively treated. As per a report by CMS in December 2024, in 2023, U.S. healthcare spending increased by 7.5%, reaching USD 4.9 trillion, which amounts to approximately USD 14,570 per person. Health expenditures accounted for 17.6% of the nation's gross domestic product (GDP). Such a continuing rise in health spending points to increasing demand for prescription therapy, including corticosteroids such as triamcinolone. Furthermore, ongoing innovations in drug formulations and delivery methods are expected to further enhance market growth in the coming years.
The triamcinolone market in Canada is expected to grow steadily due to improved access to healthcare among urban and rural communities, increased incidence of skin and allergic diseases among the aging population, availability of generic triamcinolone at very cheap prices, and government policies under Canada's public system of universal healthcare, ensuring that all patients have equal access to prescription drugs, including corticosteroids. Current partnerships have existed between dermatology companies and governmental health institutes to increase the efficiency of the supply chain and provide the availability of crucial dermatologic treatments. Increasing public awareness of healthy skin and subsequent consultations with dermatologists have sustained the demand for injectable and topical corticosteroids.
Current Health Expenditure in North America
|
Country |
Year |
Current Health Expenditure (% of GDP) |
|
U.S. |
2022 |
16.5 |
|
Mexico |
2022 |
5.7 |
|
Canada |
2023 |
11.2 |
|
Guatemala |
2022 |
7.4 |
|
Haiti |
2022 |
3.2 |
|
Cuba |
2022 |
11.7 |
Source: World Bank Group 2025
Asia Pacific Market Insights
The triamcinolone market in the Asia Pacific is expected to grow at the fastest‑rate within the forecast period due to the increased healthcare expenditure and infrastructure development, high growth of the patient base for dermatological and chronic diseases, and increasing penetration of generic and OTC (over-the-counter) corticosteroid treatments. Governments such as India and China are accelerating the availability of low-cost medicines via programs such as Ayushman Bharat and China's National Essential Drug List. Public health drives and rural outreach programs are boosting diagnosis and awareness of skin diseases and consequently further fueling demand for corticosteroids. Besides, increased domestic and foreign investment by drug firms is increasing regional manufacturing and distribution capacities.
The triamcinolone market in China is expected to grow steadily due to growing universal health insurance coverage, reduced drug prices and enhanced access, and consistent investment and entries by local and foreign drug companies. As per a report by NLM in July 2022, China has finished six rounds of centralized drug purchases and saved more than 260 billion yuan (CNY) (USD 36.4 billion) in drugs for the nation. With an average price cut of 53%, the winning bid drug volume accounted for 30% of the total annual purchases made by public medical institutions. The measures go beyond simply improving patients' access to corticosteroids, such as triamcinolone, at urban and rural hospitals.
The triamcinolone market in India is expected to grow steadily due to the massive generic drug production base ensuring extensive availability, improvement in infrastructure under public health programs, enhancing the coverage of treatment, and rising patient and practitioner awareness levels regarding skin ailments. As per a report by IBEF August 2025, the government has allocated Rs. 99,858 crore (USD 11.5 billion) to the health sector for development in the Union Budget 2025-26, a 9.78% increase from the previous allocation of Rs. 90,958 crore (USD 10.4 billion). This increased investment is expected to enhance healthcare infrastructure and accessibility, further augmenting the growth of corticosteroid treatments such as triamcinolone in India.
Europe Market Insights
The Europe market is expected to grow significantly within the forecast period due to its advanced, elaborate healthcare system having supportive reimbursement policies, growing prevalence of skin and chronic diseases among the geriatric populations, and robust pharmaceutical R&D pushing advanced corticosteroid products. Government initiatives such as the European Medicines Agency's (EMA) simplified approval regimes also fuel exposure to advanced and superior corticosteroid treatments. Public-private collaborations between private business entities and public health organizations are also improving patient education and disease management initiatives in target markets.
The triamcinolone market in the UK is expected to grow steadily due to its publicly funded National Health Service ensuring broad access to prescription corticosteroids, growing demand from rising rates of eczema and allergic conditions, and continuous focus on dermatological care quality and awareness. As per a report by ONS May 2024, Government expenditure is the principal mode of healthcare financing in the UK and stood at £239 billion (USD 322.8 billion) in 2023, representing 81.9% of overall healthcare spending. Such a strong public financing regime ensures that treatments such as triamcinolone are widely available and affordable.
The German market is expected to grow due to the presence of high healthcare expenditure and advanced medical infrastructure. The high investments in pharmaceutical R&D and innovation are also providing an enabling environment for generic and branded drug manufacturing in the country for a greater availability of treatments. Initiatives to fast-track drug approvals and reimbursements also help increase patient access to corticosteroid therapies. Collaborative partnerships between research entities and pharmaceutical firms have catalyzed innovations involving new formulations and novel delivery mechanisms.
Key Triamcinolone Market Players:
- Johnson & Johnson
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Teva Pharmaceutical Industries
- Mylan (Viatris)
- Sanofi
- Bristol-Myers Squibb
- Novartis
- GlaxoSmithKline (GSK)
- Merck & Co.
- Pfizer
- Sun Pharmaceutical Industries
- Dr. Reddy's Laboratories
- Lupin Limited
- Zydus Cadila
- Almirall
- Bayer
The global market is facing huge competition among the leading players. The major players, Johnson & Johnson, Teva Pharmaceutical Industries, and Mylan (now Viatris), leverage their broad product lines and global distribution systems to gain market share. These companies now actively invest in mergers and acquisitions, partnerships, or R&D as part of their strategy to fortify their market position. An example is Sanofi partnering with Sandoz to develop and commercialize generic triamcinolone formulations, hence fast-tracking corticosteroid treatments worldwide. Moreover, a rising demand for OTC triamcinolone products has led companies to extend their portfolio of offerings to satisfy consumer demands. Product innovation and ensured affordability remain paramount with regard to creating the competitive climate of the market.
Here is a list of key players operating in the global market:
Recent Developments
- In October 2024, Harrow (Nasdaq: HROW) has relaunch TRIESENCE (triamcinolone acetonide injectable suspension) 40 mg/mL in the U.S., restoring access to the FDA-approved, preservative-free corticosteroid for vitrectomy and ocular inflammation through a globally coordinated supply chain effort.
- In March 2022, Bausch + Lomb and Clearside Biomedical launched XIPERE, the first FDA-approved triamcinolone acetonide therapy for suprachoroidal use, treating macular edema linked to uveitis. The innovative injection targets inflammation, offering new hope to 300,000 affected Americans.
- Report ID: 8138
- Published Date: Sep 30, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Triamcinolone Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




