Pediatric Perfusion Products Market Outlook:
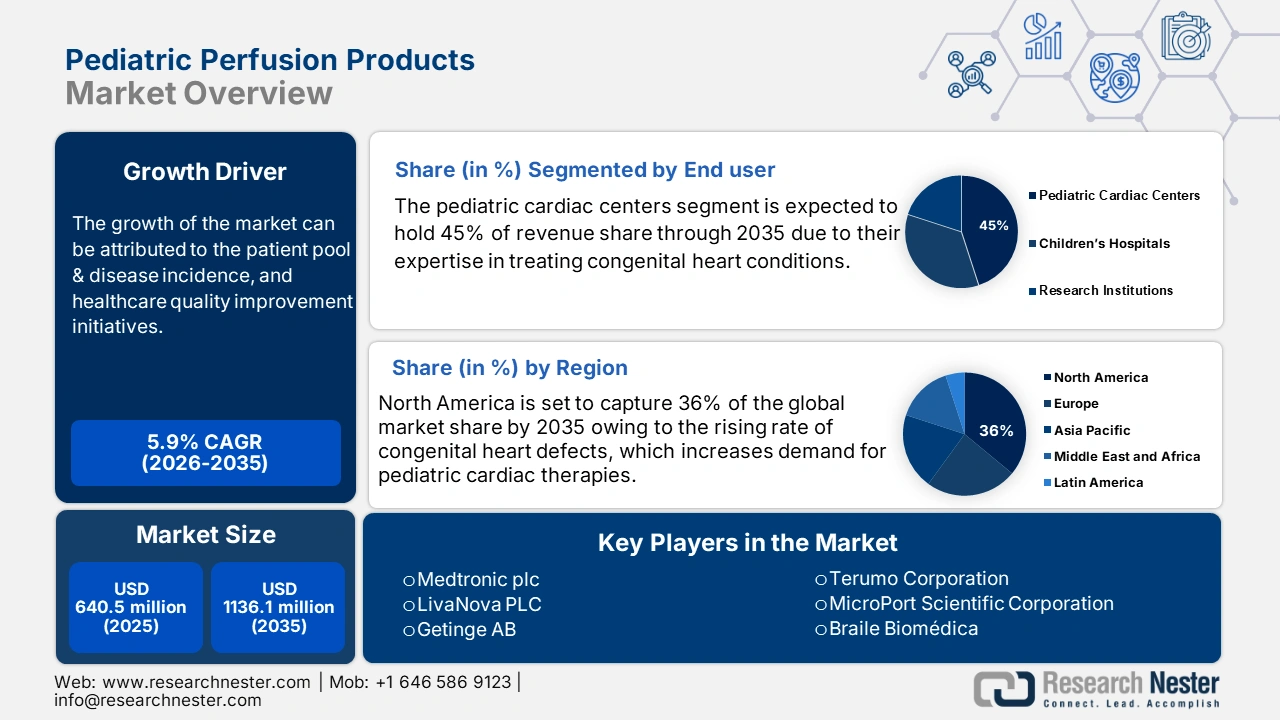
Pediatric Perfusion Products Market size was valued at USD 640.5 million in 2025 and is projected to reach USD 1136.1 million by the end of 2035, rising at a CAGR of 5.9% during the forecast period, i.e., 2026-2035. In 2026, the industry size of pediatric perfusion products is assessed at USD 678.2 million.
The pediatric perfusion products market is underpinned by a significant and stable patient pool, primarily consisting of infants born with congenital heart defects. The CDC data for October 2024 states that every 15 minutes, a child is born with a heart defect in the U.S. Hence, there will always be a need and demand for perfusion products. Trends in R&D funding indicate greater motivation from the federal government to support pediatric indications. The Pediatric Medical Device Safety and Improvement Act now allows for financial profit and priority pediatric PMA and HDE approval. Furthermore, the FDA has waived the fees and proposed a post-market registry as a way to incentivize pediatric-based information.
Investment in research, development, and deployment (RDD) is critical for advancing product safety and efficacy. Public funding agencies like the National Institutes of Health (NIH) allocate substantial grants to pediatric medical device development. On the trade side, the assembly of pediatric perfusion systems often involves a multi-national process. The raw materials or the components are sourced from various countries. This is impacted in trade data, where U.S. exports of medical equipment in 2024 were USD 27,058 million, indicating a strong international market for advanced medical technology, as per the Censes report in July 2025. On the other hand, the costs of the materials, R&D investment, and regulatory compliance have an impact on the pricing of these life-sustaining products.