Oncolytic Virotherapy Market - Regional Analysis
North America Market Insights
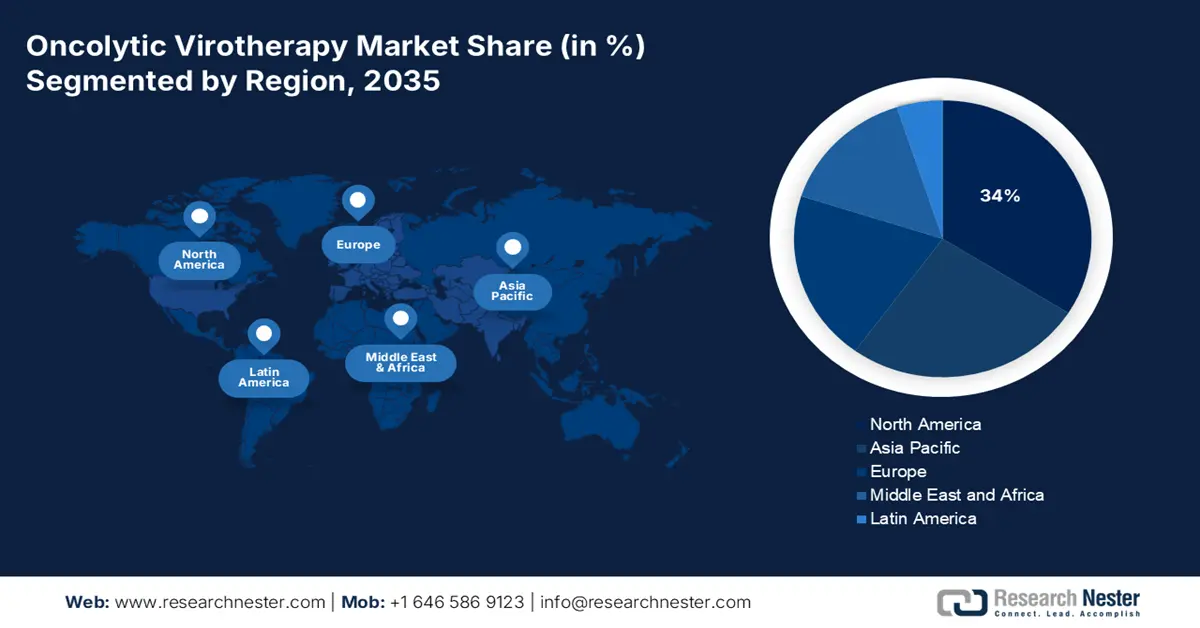
North America is expected to capture the highest share of 34% in the global oncolytic virotherapy market by the end of 2035. The growth is driven by the presence of a robust biotechnology ecosystem, regulatory support. The North American biotechnology sector, and the U.S. FDA have accelerated oncolytic virus innovation by granting fast-track designations, orphan drug approval, and support for genetically engineered biologics. For instance, according to the National Cancer Institute, the Food and Drug Administration (FDA) has approved T-VEC, an immunotherapy that uses one oncolytic virus, to treat metastatic melanoma. Although numerous are undergoing clinical trials, oncolytic viral treatments for other cancer types have not yet received approval. North American biotechnology will remain innovative and aggressive towards clinical deployment of complex biologics.
The U.S. positioned to be the lead in the market from having a multi-faceted biotech and pharmaceutical ecosystem. The country is home to a multitude of innovative companies progressing on developing advanced oncolytic virus therapeutics. The heavy investments for innovating in oncology research, levels of clinical trials, and oncolytic virotherapy’s complementary pathology with immunotherapy continually create a strong foundation for robust development and research. Additionally, the U.S. has a high level of adoption for early availability of drugs.
Canada's rise in the market is not coincidental, as the country has a beneficial backdrop of increased government funding for life sciences and cancer research. Moreover, Canada has universal healthcare and a developed clinical basis that supports the opportunity to conduct trials and safely deliver more complex biologic therapies. A 2023 report by the Fraser Institute states that in terms of the number of doctors per 1000 population, Canada came in at number 28 out of 30 nations with universal health care. In this context, we see an encouraging trend in Canadian biotech companies making virotherapy innovation a priority, often with a cooperative approach with the academic centres to speed up this process of development.
APAC Market Insights
The market in the Asia-Pacific region is anticipated to experience substantial growth by 2035. Thee growth can be attributed to the increased advancements in biotechnology, more cases of cancer, and regulatory reforms in larger countries. The region is experiencing a transformation in oncology with the increased interest in personalized and immune-based therapies. The Asia-Pacific also benefits from a diverse and relatively large patient population, which allows for comparatively easier and less expensive clinical trials. Investment in advanced healthcare by government agencies and hospitals allows for the implementation of virotherapies. For instance, India Investment Grid lists 1147 healthcare investment projects in India totalling USD 31.75 billion across all states.
The rapid growth of India’s oncolytic virotherapy market is led by the increasing healthcare investments and advances of regulatory reforms to/target clinical trials and drug approvals on time. This evolution facilitates the stepwise process of bringing new forms of therapies to market/commercialization, as this growth, given its larger cancer patient population, is expanding rapidly, and healthcare is often limited. Indeed, access to better awareness, aches for/ cravings medicine are increasing to advance healthcare/stage, which all offer India’s market as accommodating. Urban healthcare infrastructure is finding robust change to deliver and access to the complex, sophisticated therapies being developed. India's partnership with westernized biotech serves to increase innovation, technology transfer and increasing India's footing as a spontaneous and trusted nation and brand.
China is poised to lead the oncolytic virotherapy market due to its significant levels of government support for biotechnology and healthcare innovation, and relatively low barriers to entry in the marketplace. In addition, with recent regulatory changes such as accelerated approval pathways and loosened regulations for biologics, the time it takes to get a new therapy to market has been drastically reduced. China's large population also means there are numerous individuals for clinical trials and therapies. Additionally, as the government has begun to explore personalized medicine and immunotherapies, funding in oncolytic virotherapy has opened up. Domestic biopharmaceutical companies, academic institutions, and international collaborators, are working together to generate ongoing collaboration and development of oncolytic virus-based therapies.
Europe Market Insights
The oncolytic virotherapy market in Europe is expected to continue to grow steadily through 2035, given the several common factors that create an environment conducive to innovation, clinical advancement, and market adoption. Europe is home to several prominent biotech hubs, which make virotherapy R&D investments publicly and privately supported. Notably, many European regulatory bodies have created adaptive frameworks for the assessment of advanced therapy medicinal products (ATMPs). This regulatory clarity benefits all innovative continents, as it will result in pursuing the development and commercialization of virotherapies in a process for viropharmaceutical benefactor startups and large pharmaceutical companies. Further to these collaborative biopharmaceutical advancements, oncolytic virotherapy also benefits from many European cancer research networks.
Germany is distinguished by a strong pharmaceutical industry. Germany is strong on precision medicine and advanced treatment protocols for cancer. This is making it well-positioned to adopt leading-edge practices like oncolytic virotherapy. Strong healthcare systems in the country ensure patient access to new treatments. Participating in international clinical trials that foster the use of any innovative strategy leads to the diffusion and early adoption of such therapies in Germany.
Due to a vibrant biotech ecosystem and ongoing funding in cancer research, France is poised to establish a sizable oncolytic virotherapy market. The French National Agency for Medicines and Health Products Safety (ANSM) actively promotes access to emerging therapies through various supportive regulatory pathways. With France's universal healthcare system, innovative cancer treatments can be administered to broad patient populations. A collaborative environment between industry and research institutions. This aids in the clinical development and uptake of virotherapy.