Mucopolysaccharidosis (MPS) Treatment Market Outlook:
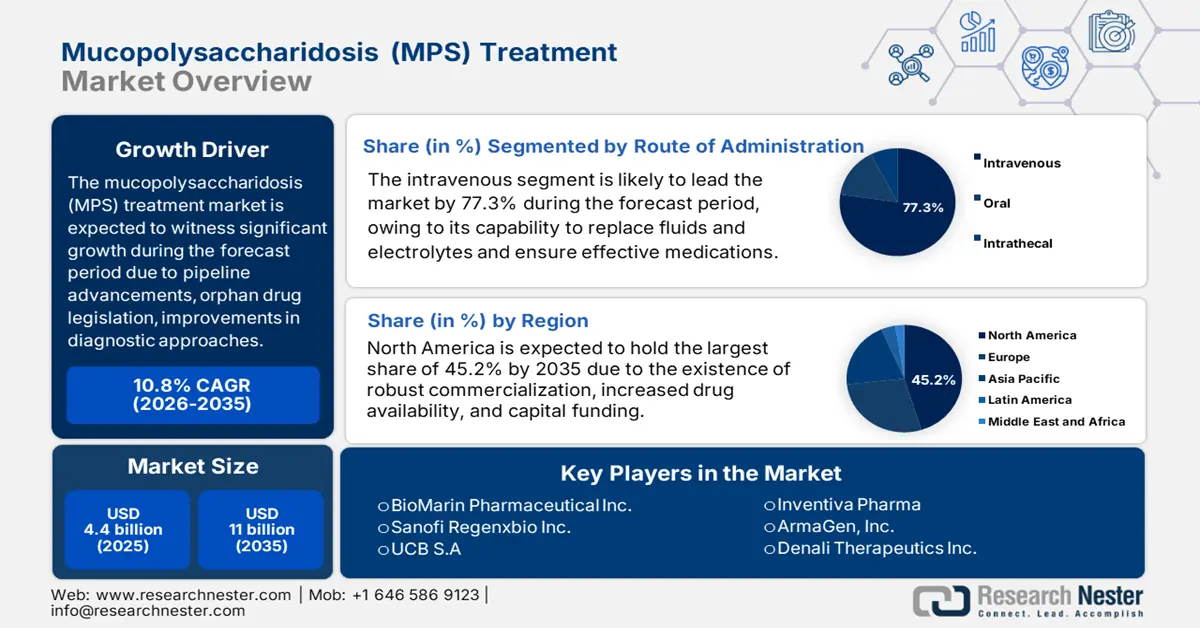
Mucopolysaccharidosis (MPS) Treatment Market size was USD 4.4 billion in 2025 and is anticipated to reach USD 11 billion by the end of 2035, increasing at a CAGR of 10.8% during the forecast period, i.e., 2026-2035. In 2026, the industry size of mucopolysaccharidosis (MPS) treatment is estimated at USD 4.8 billion.
The growth factors for the mucopolysaccharidosis treatment market are readily distilled into a core tactical framework. These factors include advancements in therapeutics, innovation in pipeline treatments, suitable orphan drug legislation, economic models, and optimization in diagnostic pathways. According to an article published by MDPI in March 2025, an estimated 2% of the overall 274,000 clinical trials were conducted in Africa, and in 2022, over 2,000 gene therapies were successfully focused on conditions, such as cardiovascular, hematological, neurological, and oncological diseases. This effectively highlights increased research efforts pertaining to treatment provision, owing to which the market is gaining increased exposure internationally.
Moreover, extension in emerging markets, tactical industrial consolidation, the presence of national institutes of health, administrative clearances, and national organization for rare diseases are also driving the mucopolysaccharidosis treatment market globally. As per the October 2022 NLM article, an estimated 6,000 to 8,000 rare diseases have been identified, with approximately 80% being genetic and 50% to 75% being pediatric onset. Besides, the EU Regulation defined this disease category as conditions severely affecting more than 50 per 100,000 patients in Europe, while the Orphan Drug Act in America defined it as impacting more than 200,000 people in the U.S., thereby bolstering the market demand.