Global Market Size, Forecast, and Trend Highlights Over 2025-2037
Materiovigilance Market size was over USD 83.4 billion in 2024 and is estimated to reach USD 214.7 billion by the end of 2037, expanding at a CAGR of 8.2% during the forecast timeline, i.e., 2025-2037. In 2025, the industry size of materiovigilance is evaluated at USD 90.2 billion.
Materiovigilance systems have gained immense importance for the investigation, evaluation, and reporting of contrary incidents related to medical devices. Besides, the adoption and implementation of medical devices are continuously rising across nations, which driving the need for the materiovigilance market globally. According to the 2025 MedTech Association Organization report, the U.S. is the largest market for medical devices, including more than 40% of the worldwide medtech market. Therefore, with such widespread, there is a huge demand for materiovigilances to effectively monitor them to avoid uncertain events.
Furthermore, the evolution of the materiovigilance market highly depends upon the continuous export and import of medical instruments in both developed and developing countries. As per the 2023 OEC report, the world valuation of medical instruments is USD 167 billion, and it is the 17th most-traded product with a complexity of 0.6. In addition, the top exporter as well as importer of medical instruments is the United States with a valuation of USD 34.8 billion and USD 37.7 billion. Therefore, the increased and easy availability of this product is a huge driving factor for materiovigilance systems internationally to detect and combat any faults to support patient safety.
Medical Instruments Export/Import
|
Countries |
Export |
Import |
|
Germany |
USD 18.4 billion |
USD 13.1 billion |
|
Mexico |
USD 17.6 billion |
|
|
China |
USD 12.3 billion |
USD 10.6 billion |
|
Netherlands |
USD 9.3 billion |
USD 14.1 billion |
|
Japan |
|
USD 6.4 billion |
Source: OEC 2023

Materiovigilance Sector: Growth Drivers and Challenges
Growth Drivers
-
Advancement and innovation in technology: The ongoing development in technology including data analytics and artificial intelligence (AI) is relatively driving the materiovigilance market globally. As per the September 2023 NLM article, there was the provision of AI to gather echocardiogram and ECG data from 52,000 patients in a clinical study. The study denoted the accuracy rate of AI to be 85.7%, 86.3% of sensitivity, and 85.7% of specificity. Therefore, the implementation of such technologies ensures suitable and standard analysis, data processing, and detection of patterns, thus positively impacting the market.
-
Rising occurrence of adverse situations: The enhancement in the prevalence of opposing incidents while handling medical devices is an effective factor for the upliftment of materiovigilance systems internationally. For instance, a study was published by NLM in February 2022, wherein the utilization rate of equipment in public referral hospitals was evaluated. It was identified that 111 equipment that is 57.8% were used in hospitals and these were received based on availability, breakdown, maintenance, and accessibility. Therefore, this results in the increasing demand for materiovigilance to cater to the identified risks to ensure patient safety.
Challenges
-
Concerns about data security: The development of anxiety when it comes to privacy and information protection constitutes a hindrance to the materiovigilance market development globally. When implementing materiovigilance systems, these manage critical product and patient information and even necessitate effective and standard cybersecurity strategies. These comprise challenges that are associated with data integrity and confidentiality that impede the nationwide adoption of materiovigilance.
-
High cost of implementation: The rise in the pricing in association with the adoption of materiovigilance systems is another challenge for market expansion. Owing to this factor, both international and regional organizations are bound to hesitate when it comes to making investments due to poor budget constraints. Particularly, small and medium-scale firms are extremely hesitant to provide funds for the expansion in the use of these systems in the healthcare sector. This eventually limits the overall penetration of technologies, thus a challenge for the market.
Materiovigilance Market: Key Insights
| Report Attribute | Details |
|---|---|
|
Base Year |
2024 |
|
Forecast Year |
2025-2037 |
|
CAGR |
8.2% |
|
Base Year Market Size (2024) |
USD 83.4 billion |
|
Forecast Year Market Size (2037) |
USD 214.7 billion |
|
Regional Scope |
|
Materiovigilance Segmentation
Mode (On-premise, Cloud-Based)
On-premise segment is poised to capture over 57.2% materiovigilance market share by 2037. This segment has gained the attention of both organizations and medical professionals owing to its control and confined accessibility. For instance, in May 2024, Rimini Street, Inc. declared that Pacific Healthcare Group has designated Rimini Support for Oracle which includes support for Oracle EBS, Oracle Technology, and Oracle Database. This will assist in operating activities such as management, distribution, and invoicing of personalized and on-premise systems, thus driving the segment’s growth.
Application (Diagnostic Application, Therapeutic Application, Surgical Application, Research Application)
By 2037, diagnostic application segment is expected to capture over 37.2% materiovigilance market share. This growth of the segment is highly driven by its vital role in ensuring the efficacy and safety of diagnostic devices and materials. According to the February 2024 Mayo Clinic Organization report, diagnosis helps in lung cancer liver survival for up to five years for at least 61.0% of the global population. In addition, this five-year survival rate for people detected with late-stage lung cancer that has spread to other areas of the body is 7%. Therefore, there is a huge need for diagnosis which includes the use of suitable medical devices, thereby driving market growth.
Our in-depth analysis of the global market includes the following segments:
|
Mode |
|
|
Application |
|
|
End Users |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Materiovigilance Industry - Regional Synopsis
North America Market Analysis
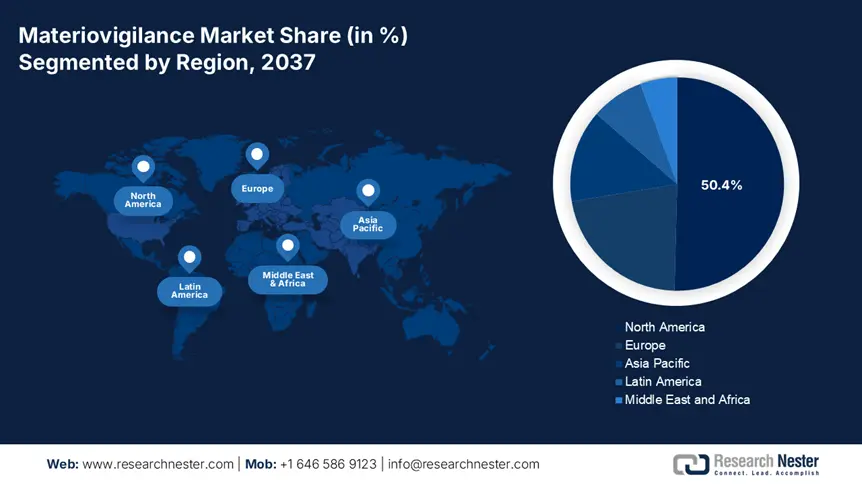
North America materiovigilance market is expected to capture revenue share of over 50.4% by 2037. The region is at the vanguard of technological revolution, with a continuous invasion of hi-tech medical technologies and devices. The incorporation of progressive materials and technologies in medical practices dictates a cautious approach to materiovigilance, further driving the demand for vigorous surveillance systems. Besides, the rising occurrence of undesirable events linked with the use of devices effectively contributes to the region’s prominence on materiovigilance systems, thus a positive outlook for market development.
The materiovigilance market in the U.S. is experiencing continuous growth, largely depending upon the presence of the U.S. FDA to approve the latest innovations in medical devices. For instance, in November 2024, Johnson & Johnson MedTech declared the U.S. FDA approval of the VARIPULSE Platform, suitable for the treatment of drug-refractory paroxysmal Atrial Fibrillation (AFib). Therefore, with such development and acceptance, medical devices are highly implemented which in turn is enhancing the need for materiovigilance systems to evaluate and monitor their functioning.
The materiovigilance market in Canada is steadily growing due to initiatives undertaken by the Government to provide ample funding in the medical sector and support healthy lifestyles. In April 2022, the Ontario government provided an investment of over USD 10 million along with USD 5 million through the Ontario Together Fund. Both these projects assisted in the upgradation of local manufacturing capacity, fast-track the commercialization of native life sciences inventions, and reinforced the province’s pandemic readiness while producing 22 new local jobs. This denotes a huge contribution to the medical technology ecosystem, thereby uplifting the market.
APAC Market Statistics
The Asia Pacific materiovigilance market is propelled to be the fastest-growing region during the forecast period. The presence and active participation of governments and administrative organizations to make the population aware of the benefits associated with the system is highly driving the market evolution in the region. Countries such as China and India, both have exceptionally displayed interest in the need for materiovigilance systems with the objective of supporting medical device efficacy and ensuring patient protection from any hazardous events during the diagnosis and treatment procedures.
The materiovigilance market in India is gaining more exposure due to the involvement of the Government to create awareness and drive demand. According to the August 2023 IPC Government report, the Government of India has approved the inauguration of the Materiovigilance Programme of India (MvPI). This comprises the inclusion of various research institutes suitable for data collection regarding unwanted events of medical devices and effectively analyze them to undertake regulatory decisions and suggest safety measures of the devices, thereby driving the increasing need and expansion of the market in the country.
The materiovigilance market in China is highly driven owing to the presence of regulatory bodies such as the National Medical Product Administration (NMPA), formerly known as the China Food and Drug Administration (CFDA). These bodies ensure the regulation and the post-approval surveillance management of medical devices. For instance, in December 2024, Mevion Medical Systems announced the NMPA approval for its MEVION S250i Proton Therapy System. This is the very first advanced and innovative technology that has been approved in the country to provide solutions to cancer patients, thus driving market expansion.
Companies Dominating the Materiovigilance Landscape
- AssurX
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Rimini Street, Inc.
- Pacific Healthcare Group
- Mevion Medical Systems
- Roche
- Sparta Systems
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems
- MDI Consultants
- QVigilance
- Qserve
- ZEINCRO
- bioAffinity Technologies, Inc.
- bioMérieux
Several organizations are playing crucial roles in shaping the materiovigilance market in the international scenario. They are implementing product innovations, service expansion, facility development, and required investments through partnerships, collaborations, mergers and acquisitions, and agreements. In January 2025, Roche stated that its whole slide imaging system, Roche Digital Pathology Dx, received an additional 510(k) clearance from the U.S. FDA. It is a scanner that provides high-resolution digitalized images to assist health providers in diagnosing cancer, thereby uplifting the market demand.
Here's the list of some key players:
Recent Developments
- In January 2025, bioAffinity Technologies, Inc. proclaimed that the Australia Patent Office accepted its patent application for the method of forecasting the probability of lung cancer by the use of CyPath lung diagnostic test for early-stage lung cancer.
- In January 2025, bioMérieux finalized the acquisition of Neoprospecta to strengthen its data and genomics offer, composed of ground-breaking pathogen and spoiler examination tools to overcome infection reinforcing its amplified diagnostics approach.
- Report ID: 7490
- Published Date: Apr 11, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Materiovigilance Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert




