Germ Cell Tumor Market - Regional Analysis
North America Market Insights
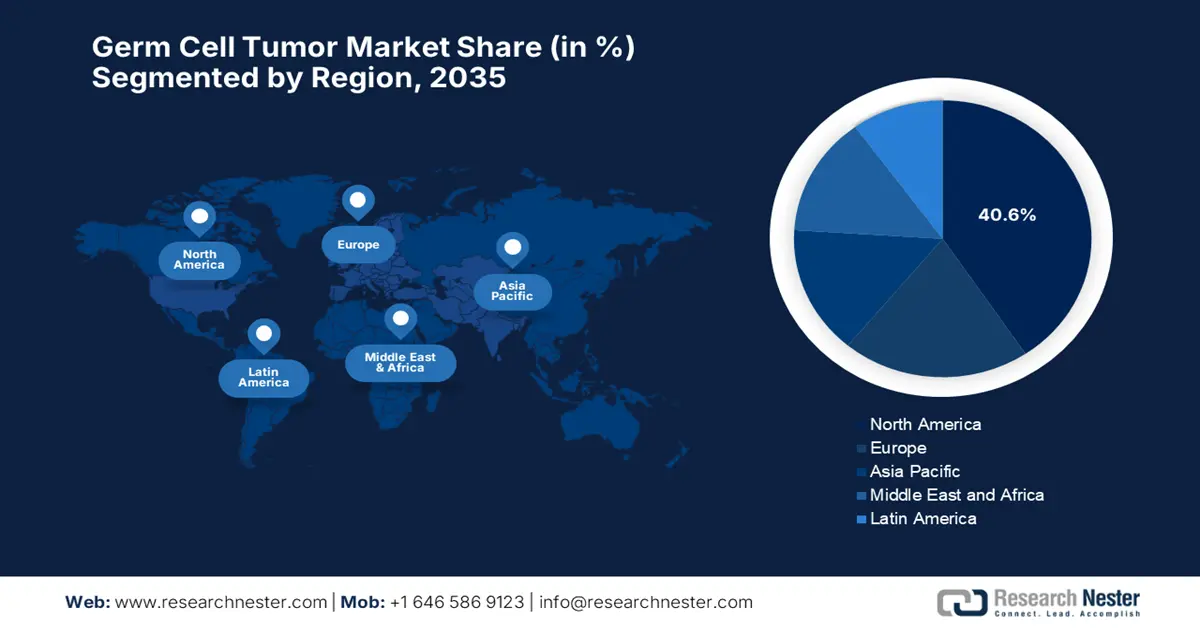
North America in the germ cell tumor market is expected to capture the largest revenue share of 40.6% by the end of 2035. The region’s upliftment in this field is readily propelled by the well-established oncology infrastructure, high awareness of rare cancers, and cross-border research collaborations. In July 2025, MiNK Therapeutics reported a complete and durable remission in a patient with metastatic, treatment-refractory testicular cancer using its allogeneic iNKT cell therapy, agenT-797. Besides, the therapy was well-tolerated with no CRS or GVHD, and donor iNKT cells remained detectable for six months. Further published in Oncogene, the case showed success after failure on platinum chemo, stem cell transplant, and multiple ICIs.
The U.S. remains a constant contributor to growth in the regional germ cell tumor market, which is successfully led by fueled by a robust pipeline of targeted therapies and expanding access to liquid biopsy technologies for tumor monitoring. NeoGenomics in July 2025 introduced PanTracer LBx, which is a blood-based comprehensive genomic profiling test for patients with advanced solid tumors. This noninvasive liquid biopsy test supports therapy selection, clinical trial matching, and monitoring, especially when tumor tissue is unavailable, hence reflecting ongoing innovation in cancer diagnostics.
Canada has gained enhanced traction in the germ cell tumor market, facilitated by the rising national investments in pediatric and rare cancer research, particularly through initiatives like the Canadian Cancer Society and government-funded genomic medicine programs. In January 2023, the country’s government readily made an investment of USD 23 million in pediatric cancer research to establish the Canadian Pediatric Cancer Consortium, which marks the largest investment in pediatric cancer research, hence a positive market outlook.
APAC Market Insights
Asia Pacific in the germ cell tumor market is considered to be the fastest-growing region between 2026 to 2035. The region’s progress in this field is subject to increasing disease awareness and access to innovative therapies. Meanwhile, countries such as Japan, South Korea, and Australia are remarkably benefiting from advanced healthcare systems that adopt global standard-of-care treatments at a rapid pace. Besides, the region is a major focus for clinical research and drug development due to its large patient population, leading to growing efforts to implement more personalized and cost-effective treatment strategies.
China is rapidly evolving in the germ cell tumor market owing to the ongoing healthcare reforms and robust domestic biomedical research and development. Also, there has been an increasing involvement in multinational clinical trials to bring novel therapies to its population. In November 2023, Grand Pharma reported that its ARC01, an mRNA therapeutic tumor vaccine using liposome nanoparticle delivery and TriMix immune-adjuvant technologies, had received acceptance for a Phase I clinical trial in China, encouraging more players to establish their footprint in the country.
India is portraying steady growth in the germ cell tumor market, extensively attributed to a high incidence rate of testicular cancers and constant government efforts to improve cancer care. For instance, in August 2025, the National Cancer Grid of India conducted its 2025 annual meeting at Tata Memorial Hospital, with participation from over 300 cancer leaders, researchers, and advocates from India and 15 other countries. The NCG, funded by India’s Department of Atomic Energy, coordinates more than 380 centers treating 60% of India’s cancer patients on a yearly basis. Notably, NCG secured an average 85% discount on cancer drug prices through negotiations with pharmaceutical companies, significantly reducing treatment costs.
Oncology Clinical Trials Registered in India (2021-2023)
|
Year |
Number of Studies (No.) |
|
2021 |
385 |
|
2022 |
414 |
|
2023 |
268 (up to June) |
Source: ASCO
Europe Market Insights
Europe is likely to retain its position as the second-largest stakeholder in the germ cell tumor market, which is driven by ongoing advances in treatment and improved survival rates. The region also benefits from platinum-based therapies and increasing healthcare investments. In April 2025, Nouscom announced positive Phase Ib/II results for NOUS-209, which is an off-the-shelf immunotherapy that targets tumors with mismatch repair deficiency and microsatellite instability by training the immune system to attack cancerous and precancerous cells. Further, these results support advancing NOUS-209 toward a registration-enabling cancer interception study.
Germany hosts a dynamic landscape of the germ cell tumor market supported by its strong research capabilities. The country also has a relatively higher incidence of testicular cancer, thereby driving consistent demand for effective diagnostic and treatment options. In October 2023, BioNTech presented positive Phase 1/2 data for its CAR-T cell therapy candidate BNT211 targeting Claudin-6 (CLDN6) in advanced solid tumors at ESMO Congress 2023. Results showed an overall response rate of 59% and a disease control rate of 95%, with prolonged CAR-T cell activity in patients receiving CARVac, hence positively impacting market growth.
The germ cell tumor market in Switzerland is also growing at a notable pace due to its huge emphasis on precision medicine and a robust clinical research ecosystem, which supports ongoing development and adoption of innovative therapies. In October 2023, Hedera Dx launched a streamlined liquid biopsy solution that enables hospitals to perform blood-based cancer testing locally, and it deliberately increases access to precision oncology by allowing faster, less invasive tumor profiling for broader patient populations. Further, the platform combines lab reagents with software, making advanced cancer diagnostics more accessible, hence a positive market outlook.
Market Landscape for Germ Cell Tumor Therapies in Europe 2024
|
Factor |
Key Findings |
|
Drug Approval |
All medicines follow one EU-wide (EMA) approval process. |
|
Key Medicines |
The market relies on older, generic chemotherapies (e.g., cisplatin). |
|
Cost of Treatment |
Generic prices can be >90% lower than original brands. |
|
Access Time |
Varies by country: <100 days (Germany) to >3 years (Cyprus, Latvia). |
Source: OECD