Compartment Syndrome Monitoring Devices Market Outlook:
Compartment Syndrome Monitoring Devices Market size was over USD 233.37 million in 2025 and is anticipated to cross USD 513.24 million by 2035, witnessing more than 8.2% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of compartment syndrome monitoring devices is assessed at USD 250.59 million.

The global evolution of the compartment syndrome monitoring devices market focuses on the manufacturing, innovation, and distribution of medical devices that are standard for the evaluation of pressure within the body’s sections. The purpose of these devices is useful for the identification of compartment syndrome that tends to disrupt tissues, reduce blood flow, and create compression in nerves. Therefore, the adoption of these monitoring devices assists in the prevention of these symptoms and promotes healthy conditions for patients.
The growth of the compartment syndrome monitoring devices market depends upon the continuous export and import of tools that are essential for the proper functioning to achieve suitable results. A few essential tools such as medical instruments, video displays, and data processing parts are readily traded globally. As per the 2023 OEC report, the global trade valuation of medical instruments is worth USD 167 billion with the United States being both the exporter and import at USD 34.8 billion and USD 37.7 billion. In another report, the overall trade of video displays is USD 70.8 billion with China being the top exporter at USD 22.4 billion and the United States being the top importer at USD 17.3 billion.
Furthermore, the 2023 OEC report also stated the world trade valuation of data processing parts and accessories to be USD 253 billion. In addition, the top exporter of this tool is China with an estimated worth of USD 87.6 billion, and the United States as the top importer at USD 54.2 billion. Besides, the product complexity of instruments is 0.6, video displays is 0.1, and processing parts is 0.9, all of which are effectively and efficiently contributing towards amplifying the compartment syndrome monitoring devices market globally. Therefore, the increased availability of these tools caters to the successful manufacturing of monitoring devices that can be used for treatment purposes of compartment syndrome.
Export/Import Comparison of Monitoring Device Tools
|
Countries |
Medical Instruments |
Video Displays |
Data Processing Parts and Accessories |
|||
|
|
Export |
Import |
Export |
Import |
Export |
Import |
|
Germany |
USD 18.4 billion |
- |
- |
USD 3.4 billion |
- |
USD 14.9 billion |
|
Mexico |
USD 17.6 billion |
- |
USD 12.7 billion |
USD 2.8 billion |
- |
USD 15.8 billion |
|
China |
USD 12.3 billion |
- |
- |
- |
- |
USD 23.3 billion |
|
Netherlands |
USD 9.3 billion |
- |
- |
- |
- |
- |
|
Vietnam |
- |
- |
USD 4.6 billion |
- |
USD 16.6 billion |
- |
|
Poland |
- |
- |
USD 3.8 billion |
- |
- |
- |
|
Slovakia |
- |
- |
USD 2.9 billion |
- |
- |
- |
|
United Kingdom |
- |
- |
- |
USD 3.2 billion |
- |
- |
|
France |
- |
- |
- |
USD 2.8 billion |
- |
- |
|
Taipei |
- |
- |
- |
- |
USD 29.7 billion |
- |
|
Thailand |
- |
- |
- |
- |
USD 21.4 billion |
- |
|
South Korea |
- |
- |
- |
- |
USD 18 billion |
- |
|
Hong Kong |
- |
- |
- |
- |
- |
USD 22.8 billion |
Source: OEC 2023
Key Compartment Syndrome Monitoring Devices Market Insights Summary:
Regional Highlights:
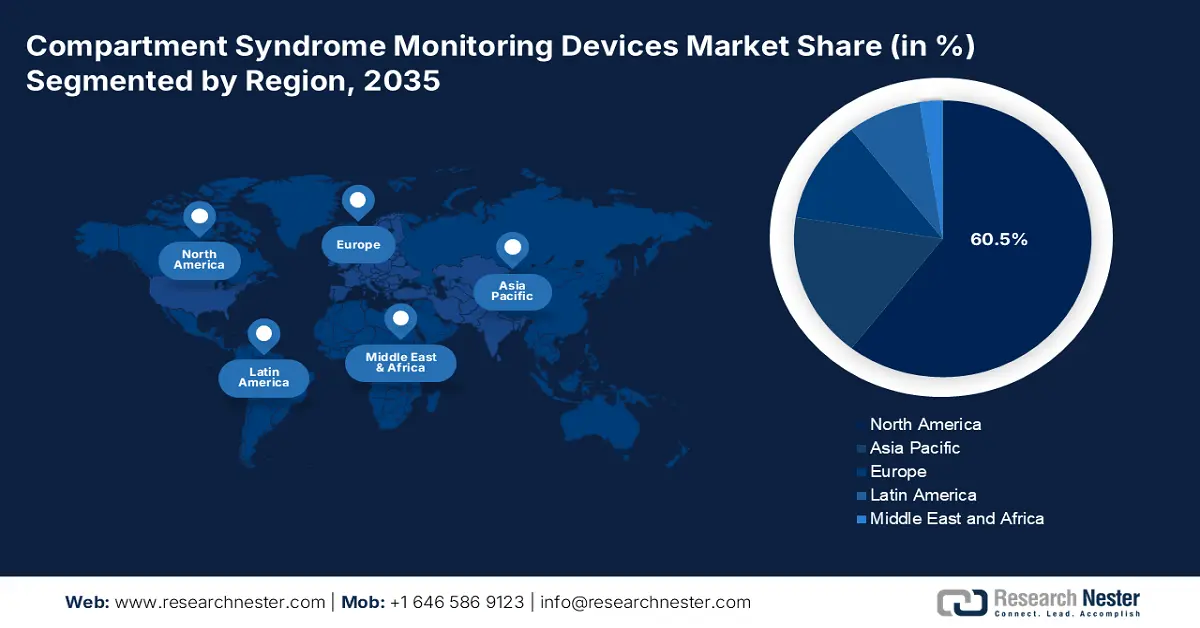
- North America dominates the Compartment Syndrome Monitoring Devices Market with a 60.5% share, driven by trauma-related conditions and healthcare expenditure, ensuring robust growth through 2026–2035.
- The compartment syndrome monitoring devices market in Asia Pacific is experiencing the fastest growth by 2035, driven by rising awareness and adoption of advanced monitoring tools.
Segment Insights:
- The Hospitals segment is anticipated to capture 50.8% market share by 2035, propelled by hospitals' critical role in early diagnosis and post-operative management.
Key Growth Trends:
- Immense focus on patient safety
- Rising awareness of healthcare professionals
Major Challenges:
- Rising cost of advanced monitoring devices
- Continuous maintenance and calibration
- Key Players: Becton, Biometrix Ltd. (3i Group), Critical Care Diagnostics (C2Dx), Inc., ConvaTec Group, Dickenson and Company.
Global Compartment Syndrome Monitoring Devices Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 233.37 million
- 2026 Market Size: USD 250.59 million
- Projected Market Size: USD 513.24 million by 2035
- Growth Forecasts: 8.2% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (60.5% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, China, Japan, Germany, United Kingdom
- Emerging Countries: China, India, Japan, South Korea, Singapore
Last updated on : 12 August, 2025
Compartment Syndrome Monitoring Devices Market Growth Drivers and Challenges:
Growth Drivers
- Immense focus on patient safety: The facilitation of pro-active care from monitoring devices to combat compartment syndrome constitutes a positive impact on patient protection, which is driving the compartment syndrome monitoring devices market globally. As per the September 2023 WHO report, 1 in every 10 patients is wounded in healthcare and over 3 million deaths happen yearly due to unsafe care. To avoid this situation, these devices are designed in a way to accurately recover trajectories and provide better results by preserving optimal compartment compressions.
- Rising awareness of healthcare professionals: Medical care providers are increasingly becoming aware of the compartment syndrome globally, which is yet another growth factor for the compartment syndrome monitoring devices market internationally. Besides, as per the August 2024 U.S. Bureau of Labor Statistics, the proportion of athletes and sports as an occupation is expected to increase by 11% by 2033. This category of people is highly subjected to suffering from the syndrome owing to which the market is expanding rapidly and so is the awareness of physicians and other healthcare providers.
Challenges
- Rising cost of advanced monitoring devices: The pricing of the latest and advanced monitoring devices is hindering the upliftment of the compartment syndrome monitoring devices market. The aspect of reimbursement rates and technological costs deter both medical providers and patients from purchasing these devices owing to the presence of financial difficulties in recovering their investments. These monitoring devices require effective care, standardization, and regular cleaning, all of which are very expensive. Moreover, software upgradation, replacement of sensors, and technical support cater to recurring expenses, restraining market evolution.
- Continuous maintenance and calibration: The aspect of accuracy, adherence to guidelines, handling costs, and keeping up with technological improvements are essential for monitoring devices. If these parameters are unattained, the compartment syndrome monitoring devices market can be negatively impacted. This can result in inaccurate data, ultimately leading to inappropriate diagnosis and treatment. Also, the presence of vibration, humidity, and adverse temperature can disrupt the maintenance of these devices, thus posing a huge challenge for market expansion.
Compartment Syndrome Monitoring Devices Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
8.2% |
|
Base Year Market Size (2025) |
USD 233.37 million |
|
Forecast Year Market Size (2035) |
USD 513.24 million |
|
Regional Scope |
|
Compartment Syndrome Monitoring Devices Market Segmentation:
End Use (Hospitals, Clinics)
Hospitals segment is expected to dominate compartment syndrome monitoring devices market share of over 50.8% by 2035. Hospitals are the foremost care accommodations for innumerable health conditions including trauma injuries that may result in compartment syndrome. As per the January 2023 NLM article, there is provision of prognosis in hospital post the treatment. After the completion of the fasciotomy within six hours, the recovery rate of limb function is nearly 100%. Therefore, to guarantee a successful diagnosis and treatment procedure for patients with the condition, an initial assessment is constituted in a hospital to ensure post-operative management.
Syndrome Type (Abdominal Compartment Syndrome, Acte uCompartment Syndrome, Chronic Compartment Syndrome)
The abdominal compartment syndrome segment is expected to influence the compartment syndrome monitoring devices market at a considerable rate during the forecast timeline. This particular syndrome has become more common internationally because of numerous situations including perilous illness, sepsis, trauma, and surgery. As per an article published by Surgery in August 2024, a clinical study was conducted on 11,804,585 patients, out of which 19,644 that is 0.17% suffered from the syndrome and its incidence rate ranged between 0.1% to 0.2%. Therefore, this denotes the rapid adoption of compartment syndrome monitoring devices to get rid of the condition through proper diagnosis and treatment.
Our in-depth analysis of the global compartment syndrome monitoring devices market includes the following segments:
|
Product |
|
|
Syndrome Type |
|
|
End Use |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Compartment Syndrome Monitoring Devices Market Regional Analysis:
North America Market Analysis
The compartment syndrome monitoring devices market in North America is expecting its supremacy during the forecast timeline with a notable share of 60.5%. The occurrence of trauma-related injuries, peripheral vascular disease, and diabetes are among the chronic illnesses that massively upsurge the danger of compartment syndrome. However, monitoring tools that can support the primary diagnosis and treatment of compartment syndrome in patients with fundamental medical conditions are becoming more essential in the region. In addition, the acceptance of renowned medical technologies such as compartment syndrome monitoring devices is made possible by expenditure on healthcare which is driving the market growth.
The compartment syndrome monitoring devices market in the U.S. has gained positive exposure due to the involvement of administrative bodies in approving the launch of the latest monitoring devices. According to the September 2020 U.S. FDA report, an estimated 75% of cases of severe compartment syndrome are triggered by a fracture of the leg or arm, with tibial shaft fractures representing the most common etiology. However, to combat this, the U.S. FDA has stated the importance of intracompartmental pressure monitors to provide possible treatment solutions. These devices comprise a fluid-filled slit catheter as well as a syringe-based manometer to evaluate the resistance that exists when a minor volume of saline solution is vaccinated into the compartment.
The compartment syndrome monitoring devices market in Canada is expected to boost since the government has ensured investments for the increased availability of medical devices. For instance, in the April 2021 Government of Canada report, funding of USD 240.5 million was provided by the Prime Minister in May 2020 to enhance the accessibility of digital tools and virtual services to support regional health and well-being. Simultaneously, in May 2021, the Ontario Government invested more than USD 1.5 million through the Ontario Together Fund, in Myant Inc. The purpose was to assist in commercializing connected wearables that can sense, monitor, and detect various symptoms that may signal the onset of diseases.
APAC Market Statistics
The compartment syndrome monitoring devices market in APAC is the fastest-growing region and is expected to observe rewarding progress during the forecast timeline owing to the awareness of the condition. This has resulted in a huge necessity for monitoring tools to accurately measure and track intracompartmental pressure, allowing medical providers to make quick clinical decisions. Furthermore, policies that augment the cost-effectiveness of healthcare services while maintaining quality and accessibility recurrently integrate provisions for cutting-edge medical technologies and equipment. Therefore, all factors are readily responsible for amplifying the market in the region.
There is a positive outlook for the compartment syndrome monitoring devices market in India since compartment syndrome is a serious medical issue often arising from trauma, especially among athletes. Besides, in an article published by CONNECTCX in May 2024, the healthcare industry in the country is gaining more exposure to internet-connected IOT wearable devices that ensure actual monitoring and analysis of vital health metrics. In addition, key players such as Fitbit and Xiaomi are empowering regional consumers with perceptive data related to sleep patterns, activity levels, and heart rates. Thus, the adoption of innovative solutions in providing medical solutions is readily driving the market growth in the country.
China’s compartment syndrome monitoring devices market is developing due to improvements in healthcare organization and cumulative healthcare spending. This is possible with the adoption of devices such as the STIC Pressure Monitor (formerly by Stryker, now C2Dx) being utilized for diagnosing and managing the condition caused by the syndrome. As per the July 2020 NLM article, the distribution of medical devices in the country has increased with a growth rate of 40% owing to the introduction of China’s latest medical reform planning. Thus, with the presence of appropriate strategies, the market is expected to boost in the country based on increased investment.
Key Compartment Syndrome Monitoring Devices Market Players:
- Becton
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Biometrix Ltd. (3i Group)
- Critical Care Diagnostics (C2Dx), Inc.
- ConvaTec Group
- Dickenson and Company
- Millar, Inc.
- Medline Industries, Inc.
- Medtronic plc
- MY01, Inc.
- Potrero Medical
- Spiegelberg GmbH & Co.KG.
- Schwarzer Cardiotek
The compartment syndrome monitoring devices market is branded by the existence of numerous recognized and evolving players that offer a diversity of products across the world. The market players compete based on factors such as innovation, product quality, customer service, regulatory compliance, and pricing strategies. In addition, other factors including mergers and acquisitions, collaborations, and partnerships are also readily driving the market expansion. For instance, in August 2021, Schwarzer Cardiotek and Millar Inc. successfully operated in the form of a partnership to promote the direct connection between the Millar Mikro-Cath and the EP-TRACER recording system.
Moreover, the collective utilization of the Millar Mikro-Cath pressure catheter along with the EP-TRACER ensures improvement for the efficient embedding of pacemakers (optimal placement of the pacemaker electrode/configuration). Besides, with the implementation of this catheter, the user has the possibility to assess and cautiously monitor the contractility of the left ventricle during stimulation by the pacemaker in real-time. Therefore, such developments of organizations globally are fruitful for the emergence of the latest monitoring devices, efficiently utilized by health professionals and patients, thus an optimistic outlook for the compartment syndrome monitoring devices market.
Here's the list of some key players:
Recent Developments
- In December 2024, MY01 announced the shipment of its 2,000th device, highlighting its devotion to refining patient outcomes by providing real-time, continuous pressure monitoring that aids healthcare professionals in making timely and objective decisions.
- In April 2024, Medtronic plc received the U.S. FDA approval for Inceptiv closed-loop rechargeable spinal cord stimulator (SCS) device for the treatment of chronic pain. This can sense biological signals along the spinal cord and inevitably regulates stimulation in real-time, keeping therapy in harmony with the motions of daily life.
- Report ID: 7376
- Published Date: Aug 12, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Compartment Syndrome Monitoring Devices Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




