Blau Syndrome Market Outlook:
Blau Syndrome Market size was valued at USD 1.02 billion in 2025 and is likely to cross USD 2.53 billion by 2035, expanding at more than 9.5% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of blau syndrome is assessed at USD 1.11 billion.

The blau syndrome market is experiencing a substantial growth owing to the increase in the number of blau syndrome cases in the world, which consequently spur the number of treatment options. The prominent players in the market are advancing treatment modalities to deliver the market with utmost potential drugs to improve recovery rates and patient outcomes. For instance, in March 2025, Domain Therapeutics has reported that it has nominated and is advancing DT-9046, a candidate with "game-changing" potential across several inflammatory disease markets. It includes atopic dermatitis (AD), inflammatory bowel disease (IBD), arthritis but also neuroinflammation including migraine.
The use of corticosteroids to treat blau syndrome has been connected with the incidence of obstinate prognostics, which denotes the utilization of immunosuppressant. Due to this, suppressants are vastly employed by the patients, even though variable rates of efficacy have been observed. For instance, in December 2024, PIF Partners announced that the U.S. FDA granted Rare Pediatric Disease Designation (RPDD) to its lead investigational therapeutic 101-PGC-005 ('005) for the treatment of systemic juvenile idiopathic arthritis (sJIA) flares. '005 was tested in Phase 3 clinical trials in 9 Indian centers for the treatment of COVID-19-induced ARDS, which is registered on CTRI/2024/01/061531.
Key Blau Syndrome Market Insights Summary:
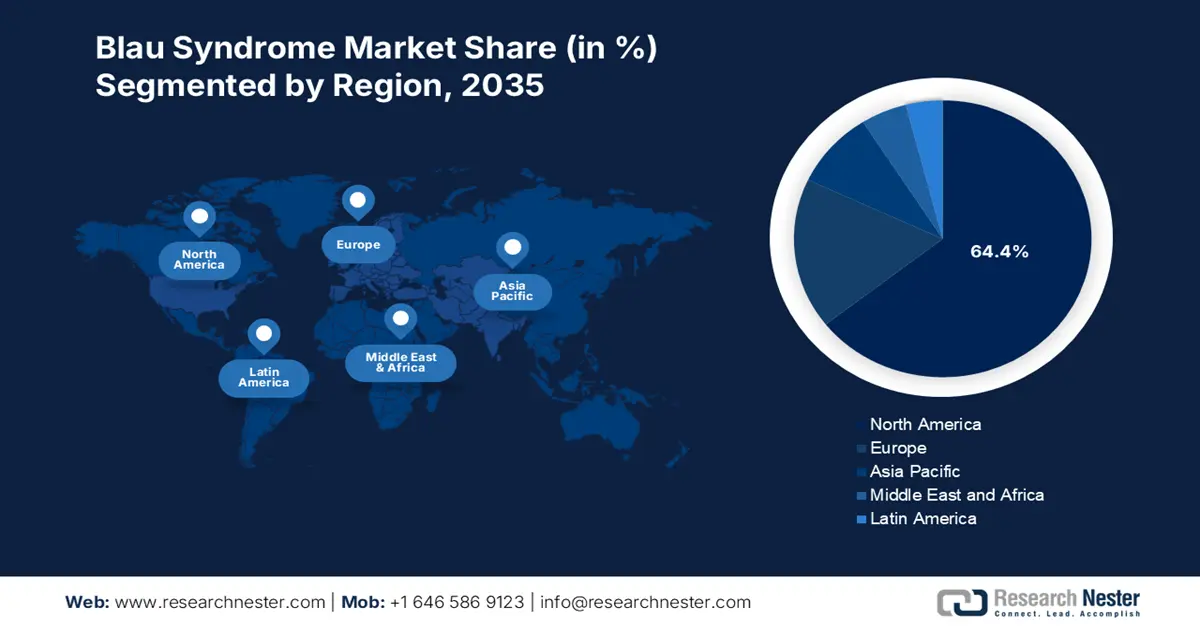
Regional Highlights:
- North America holds a dominant 64.4% share in the Blau Syndrome Market, fueled by the adoption of innovative therapies and continued R&D efforts, driving strong growth prospects through 2035.
- The Blau Syndrome Market in Asia Pacific is expected to experience the fastest growth through 2026–2035, attributed to increasing genetic studies and personalized treatment approaches.
Segment Insights:
- The Oral segment is projected to capture 52.5% market share by 2035, fueled by convenience, patient compliance, cost benefits, and growing disease incidence.
Key Growth Trends:
- Emerging biologic therapies
- Orphan designations and incentives
Major Challenges:
- Restricted understanding of disease pathogenesis
- Reimbursement and access issues
- Key Players: Novartis AG, Amgen Inc., Abbvie Inc, Alkem labs, Accord healthcare, and more.
Global Blau Syndrome Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 1.02 billion
- 2026 Market Size: USD 1.11 billion
- Projected Market Size: USD 2.53 billion by 2035
- Growth Forecasts: 9.5% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (64.4% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, United Kingdom, Japan, France
- Emerging Countries: China, India, Brazil, Russia, Mexico
Last updated on : 12 August, 2025
Blau Syndrome Market Growth Drivers and Challenges:
Growth Drivers
- Emerging biologic therapies: The prime driver in the growth of the blau syndrome market is continuous evolution of biological therapies. For instance, in December 2024, eyeDNA Therapeutics reported that the US FDA had granted HORA-PDE6b, the company's gene therapy for those with inherited retinal dystrophy (IRD) due to mutation of the PDE6b gene, Rare Pediatric Disease Designation (RPDD). These therapies demonstrate potential for application towards treatment of the characteristic manifestations of the disease, such as arthritis, uveitis, and dermatitis, and thus towards improved patient outcomes.
- Orphan designations and incentives: A key growth drivers for the Blau syndrome market is orphan drug designations and programs offered with extended market protection, lowered R&D expense, and possible grant funding. For instance, in December 2024, a research investigating the advantages of using nutritional therapy and biologic drugs together in children with Crohn's disease was awarded a USD 1 million PIONEER Grant. This grant to research recipients at University of British Columbia, making the administration of a biologic drug more effective with the inclusion of a nutritional remedy to curb inflammation.
Challenges
- Restricted understanding of disease pathogenesis: The blau syndrome market is faced with a primary hurdle based on the partially obscure pathogenesis of this orphan autoinflammatory condition. Such incomplete understanding constrains absolute targets for therapy and complicates the discovery of very specific and efficient drugs. Clinical treatment is thus mostly based on general immunosuppression normally accompanied by very strong off-target effects and unreliable efficacy. Lack of clear molecular understanding of disease incidence and development prevents the development of targeted remedies, thus preventing market expansion.
- Reimbursement and access issues: The low patient population in the blau syndrome market naturally limits commercial demand for drugs resulting in charging increased price on current treatments. Overly burdensome cost leads to significant access barriers among patients, especially in reimbursement-constrained health care systems or even in countries with overall lack of coverage for orphan diseases. In addition, the absence of general awareness and experience among clinicians in the treatment and diagnosis of Blau syndrome can lead to delayed diagnosis and initiation of treatment, further creating barriers to appropriate and timely care among affected individuals.
Blau Syndrome Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
9.5% |
|
Base Year Market Size (2025) |
USD 1.02 billion |
|
Forecast Year Market Size (2035) |
USD 2.53 billion |
|
Regional Scope |
|
Blau Syndrome Market Segmentation:
Route of Administration (Oral, Topical, Parental)
Oral segment is poised to capture over 52.5% blau syndrome market share by 2035. The growth is attributed to the intrinsic benefits such as convenience of use, patient compliance, and cost of manufacture, complemented further by the growing incidence of diseases. It therefore, requires chronic oral treatment as well as advancement in formulation science for better drug bioavailability. For instance, in October 2023, the U.S. FDA approved Agamree (vamorolone) to treat Duchenne muscular dystrophy (DMD) patients 2 years and older. It provided them with a better-tolerated, but equally effective, oral corticosteroid alternative.
Distribution Channel (Hospital pharmacy, Retail pharmacy, Online pharmacy)
The hospital pharmacy segment is anticipated to dominate the blau syndrome market throughout the projected timeframe owing to its strategic position of providing prescription drugs for inpatient and outpatient therapy. It is further supplemented by the rising number of surgery procedures, hospital admissions, and convenient accessibility of a wide range of pharmacy products within the hospital environment. For instance, in November 2022, it was unveiled by the Candian Digestive Health Foundation that, over 25% of individuals are diagnosed at less than 18 years of age, with increasing incidence of IBD in children. In addition, the frequency of IBD-specific outpatient visits following 2005 increased by 4.0% annually.
Our in-depth analysis of the global blau syndrome market includes the following segments:
|
Route of Administration |
|
|
Distribution Channel |
|
|
End user |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Blau Syndrome Market Regional Analysis:
North America Market Statistics
North America in blau syndrome market is set to hold over 64.4% revenue share by the end of 2035. The driving force behind the market growth is driven by the thorough increased use of innovative and new therapeutic modalities. Furthermore, the continued R&D efforts in this space, as well as the discovery of other biologics and targeted therapies, further support the likelihood of robust market growth during the stipulated timeline.
In the U.S. blau syndrome market is likely to unveil lucrative growth opportunities owing to the research and developmental programs conducted by the companies and research institutes. For instance, in July 2022, St. Jude Children's Research Hospital stepped up its investment in programs to further the research and treatment of pediatric cancer and other devastating diseases. The expansion includes an extra USD 1.4 billion to the institution's six-year operating and capital budget, totaling USD 12.9 billion. The growth also includes raising capital for building, rebuilding and capital requirements from USD 1.9 billion to USD 2.3 billion. The 2022–27 plan mapped out projects across the disciplines of structural biology, advanced microscopy and data sciences.
Canada blau syndrome market is exponentially increasing its footprints owing to assistance offered by the local governments through fundings and grants. For instance, in March 2025, the Governments of the Northwest Territories and Canada signed the National Strategy for Drugs for Rare Disease (DRD) agreement to commit over USD 7.8 million for three years. This commitment is made in order to enhance access to new rare disease drugs for residents and support improved access to available drugs, early screening, and diagnosis for rare diseases. In addition, in March 2023, it pledged up to USD 1.5 billion over a period of 3 years to support the same cause, including up to USD 1.4 billion for improved quality of life.
Asia Pacific Market Analysis
The blau syndrome market in Asia Pacific is likely to witness fastest growth during the stipulated timeframe attributable to the increasing emphasis on genetic studies that creates opportunities for drug companies to create new drugs and methods. In addition, a shift towards individualized treatment regimens that take into account the individual genetic profiles of patients is expected to bolster the growth. This improves the effectiveness of treatments and supports better patient outcomes.
In India blau syndrome market is likely to witnessing robust growth owing to the digital health solutions that are gaining prominence. Moreover, research and investigational studies are promoting the market share. For instance, in October 2021, Indian Institute of Technology, Kanpur (IIT-K) and The University of Queensland (UQ) released the findings of a groundbreaking international collaborative study in inflammatory disease. The research, which was published in 'Molecular Cell', that sheds new light on a protein receptor, C5aR2, that is involved in the regulation of numerous immune and inflammatory processes. In addition, it investigated its potential as a therapeutic target for the treatment of several chronic inflammatory diseases.
China blau syndrome market is likely to flourish at a fast pace during the stipulated timeline due to the smooth regulatory procedures and methods that allow to change the landscape in the treatment of such diseases. For instance, in January 2025, GSK plc reported that new drug applications were accepted for review by China's National Medical Products Administration. The indications submitted are for add-on maintenance therapy of asthma in patients aged 12 and above with adult and adolescent type 2 inflammation with blood eosinophil count as a characteristic, and add-on maintenance therapy of inadequately controlled adult patients with CRSwNP.
Key Blau Syndrome Market Players:
- Novartis AG
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Pfizer
- Salix Pharmaceuticals
- Mylan N.V
- Himca pharma plc
- ONCODESIGN
- Amgen Inc
- Abbvie Inc
- Alkem labs
- Accord healthcare
- Zydus Pharmaceuticals
- Centogene AG
- Teva Pharmaceuticals
- Amneal Pharmaceutical Inc.
The competitive players in the blau syndrome market are engaged in the process of applying the strategic growth policies to increase their market share. For instance, in April 2024, it was unveiled that Roivant Sciences' (ROIV.O) potential treatment for non-infectious uveitis, an inflammatory eye disease, reduced symptoms of the condition in a mid-stage trial, pushing the shares of the biotech company up by almost 8%. These successful commercialization of the diagnostics and drugs, capable of diagnosing the disease, and minimizing the symptoms of blau syndrome respectively, is contributing towards the development of the market during the forecast period.
Here's the list of some key players:
Recent Developments
- In April 2024, Glenmark Pharmaceuticals announces to have receive the approval for Acetaminophen and Ibuprofen tablets (250 mg/125 mg) from the US FDA to sell a generic version of anti-inflammatory drug in the American market.
- In May 2023, AbbVie announced the U.S. FDA has approved RINVOQ (upadacitinib) for the treatment of adults with moderately to severely active Crohn's disease with an inadequate response to one or more TNF blockers.
- Report ID: 7555
- Published Date: Aug 12, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Blau Syndrome Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




