Aspergillosis Treatment Market Outlook:
Aspergillosis Treatment Market size was valued at USD 4.35 billion in 2025 and is expected to reach USD 6.76 billion by 2035, expanding at around 4.5% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of aspergillosis treatment is evaluated at USD 4.53 billion.
The increasing incidence of aspergillosis infections, specifically among immunocompromised people, is primarily pushing the demand for innovative treatment techniques. Moreover, Aspergillosis can pose as a life-threatening disease so the requirement for effective treatment is rising continuously. All across the world, almost 3,000,000 cases of chronic pulmonary aspergillosis, around 223,100 cases of cryptococcal meningitis complexing HIV/AIDs, roughly 700,000 cases of intrusive candidiasis, approximately 500,000 cases of Pneumocystis jirovecii pneumonia, almost 250,000 cases of intrusive aspergillosis are reported, yearly.
The medical technology developments and drug modification will further help the aspergillosis treatment market to hike in its anticipated CAGR in the coming years. Improvements in medical technology and drug development have taken to more focused and efficient therapies, modifying patient results and treatment efficiency. Antifungal drugs are primarily utilized to cure Aspergillosis. Voriconazole, an antifungal drug is extensively employed because of its fewer side effects and more efficiency in comparison to other drugs. In the most substantial studies encircling treatment of aspergillosis, voriconazole demonstrated about 30% entire mortality on day 84, with reports of feedback rates ranging between 36% and 52.8%. The starting implementation of voriconazole was related to reduced length of hospital keeping in a sub-group assessment of the TRANSNET population. Itraconazole or Amphotericin B also cures the aspergillosis infection successfully.
Key Aspergillosis Treatment Market Insights Summary:
Regional Highlights:
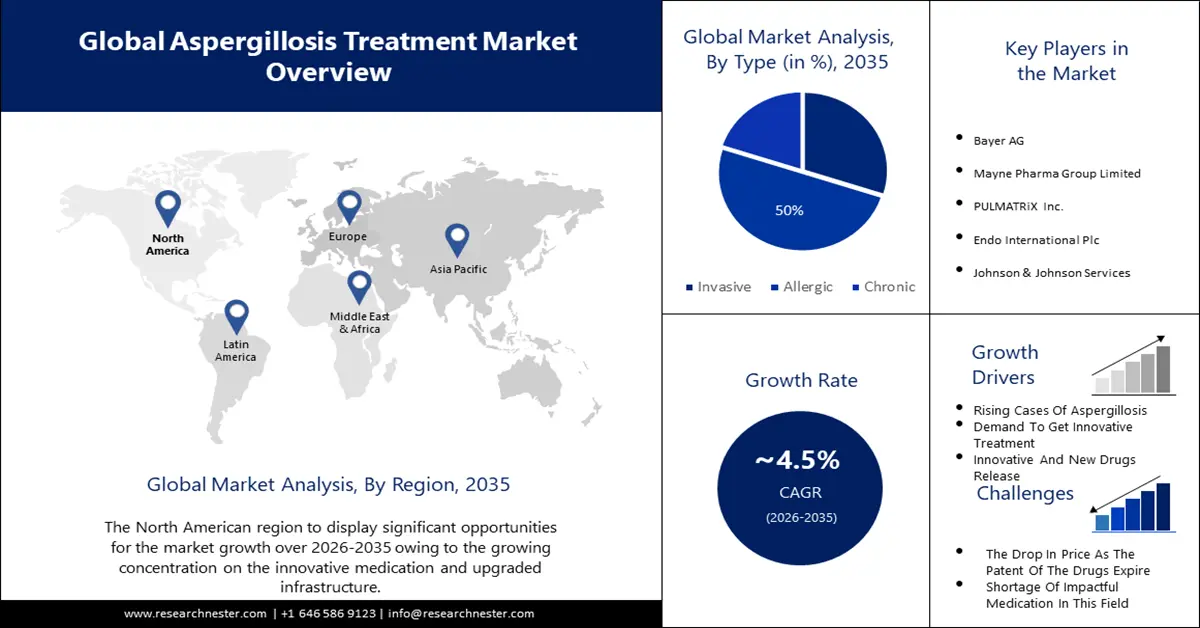
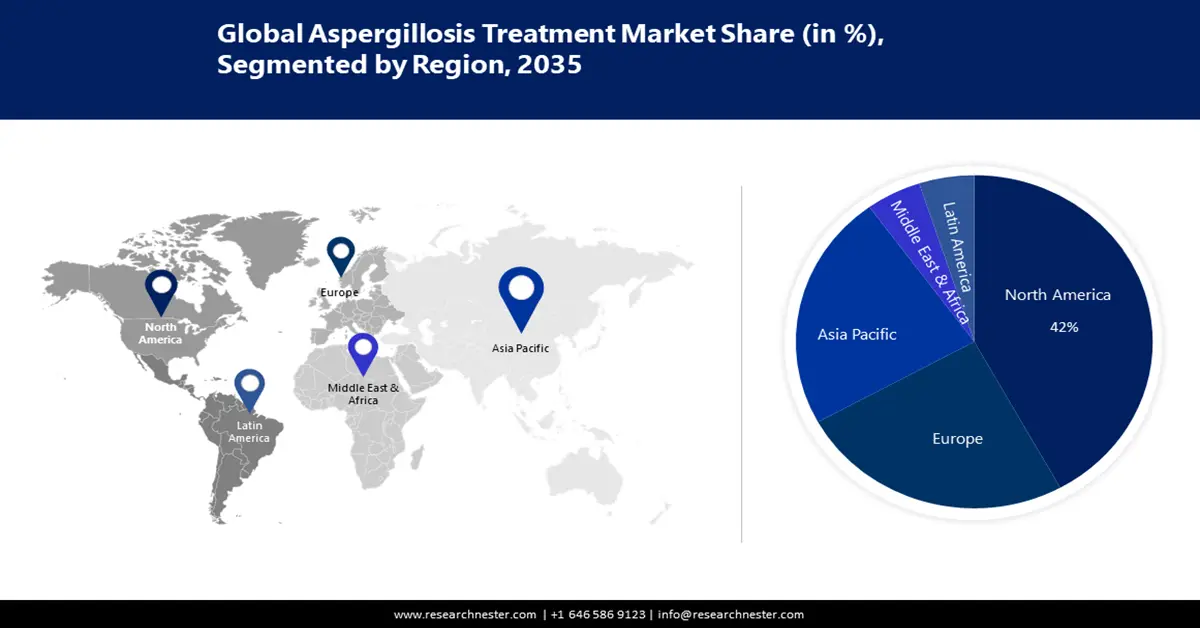
- North America aspergillosis treatment market is projected to capture a 42% share by 2035, driven by high prevalence of lung diseases, HIV, and organ transplants.
Segment Insights:
- The antifungal segment in the aspergillosis treatment market is projected to capture a 60% share by 2035, driven by the efficacy of antifungal drugs and ongoing development of focused treatments.
- The allergic aspergillosis segment in the aspergillosis treatment market is projected to see significant growth through 2035, driven by developed incidences of respiratory problems like asthma and COPD.
Key Growth Trends:
- Implementation of Several Rules to Limit Aspergillosis Infection
- Advanced and Modern Diagnostic Capabilities
Major Challenges:
- The Patent Expiry of Branded Products Drops the Price
- Lack of Latest Innovations and Technologies in Aspergillosis Treatment
Key Players: Bayer AG, Mayne Pharma Group Limited, PULMATRiX Inc., Endo International Plc, Johnson & Johnson Services Inc., GlaxoSmithKline Plc, Pfizer Inc., Abbott, Takeda Pharmaceutical Company Limited, Novartis AG, Shionogi & Co., Ltd., Asahi Kasei Pharma Corporation, F2G Ltd., Regeneron Pharmaceutical Inc.
Global Aspergillosis Treatment Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 4.35 billion
- 2026 Market Size: USD 4.53 billion
- Projected Market Size: USD 6.76 billion by 2035
- Growth Forecasts: 4.5% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (42% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, Japan, United Kingdom, China
- Emerging Countries: China, Japan, South Korea, India, Singapore
Last updated on : 11 September, 2025
Aspergillosis Treatment Market Growth Drivers and Challenges:
Growth Drivers
-
Implementation of Several Rules to Limit Aspergillosis Infection- The favorable government and organization act by different countries will create an impact on the aspergillosis treatment market as this will help create and modify the aspergillosis treatment. Moreover, Interregional Research Project Number 4 (IR–4) delivered a petition to EPA under the Federal Food, Drug, and Cosmetic Act (FFDCA), seeking to modify the existing allowance immunity for Aspergillus flavus strain AF36 for better Aspergillus treatment. This act reduces the requirement to set up an optimum allowable level for remains of Aspergillus flavus strain AF36 under FFDCA when implemented in keeping with the amended tolerance exemption.
-
Advanced and Modern Diagnostic Capabilities - The developing intelligence and the modernized approach to diagnosing the aspergillosis infection will help in more effective and prolific aspergillosis treatment. Nowadays, healthcare providers use different measures like tissue biopsy, in which way a miniature model of infected tissue is assessed in a laboratory for proof of aspergillus under a microscope or in a fungal culture. A blood test can assist in diagnosing intrusive aspergillosis early in people with massively diminished immune systems. Also, the healthcare providers ask about your medical history, risk factors, symptoms, physical investigation, and lab tests when diagnosing aspergillosis. Individuals may be required to make photo tests like a chest x-ray or a CT scan of their lungs or other parts of their body according to the place of the supposed infection.
- Increased Awareness in People - The augmented awareness about the aspergillosis symptoms and peoples’ interest in curing them are increasing day by day asking for productive aspergillosis treatment. Furthermore, due to the increased concern in people, they are more likely to rush to identify their ailment. Thus, the early identification and quick treatment initiation are further creating a surge in the need for different treatment measures.
Challenges
-
The Patent Expiry of Branded Products Drops the Price - Patents can create creativity by giving the manufacturer the scope for a part-time monopoly and a period of market particularity. Drug prices decrease substantially after patent expiry. During this period of market particularity, pharmaceutical agencies can get back the scope costs made during the drug formation technique. The authority of market particularity for new products encourages new investments in Research and Development (R&D). Multiple factors may impact the length of aspergillosis treatment market particularity, involving the moment of patent filing, the size of the R&D technique afterward, the registration technique, and time to acceptance/refund by the US Food and Drug Administration (FDA)/European Medicine Agency (EMA) and national health technology assessment (HTA) companies, and the length before acceptance of generic drugs. The extent of this price decrease differentiates massively between products and countries. It is shown that drug prices dropped substantially with drug price ratios aged between 6.6 to 66% 1–5 years after patent expiry.
-
Lack of Latest Innovations and Technologies in Aspergillosis Treatment
- Availability of Low Impact Medicines in the Market.
Aspergillosis Treatment Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
4.5% |
|
Base Year Market Size (2025) |
USD 4.35 billion |
|
Forecast Year Market Size (2035) |
USD 6.76 billion |
|
Regional Scope |
|
Aspergillosis Treatment Market Segmentation:
Type Segment Analysis
The allergic segment in the aspergillosis treatment market is anticipated to hold the largest share of over 50% by 2035. The allergic segment's revenue share in the market can be attributed to the developed incidences of respiratory problems like asthma, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, and pneumonia. In line with the National Center for Biotechnology Information, allergic bronchopulmonary aspergillosis (ABPA) impacts about 4 million people internationally. In developing countries, tuberculosis is the susceptible factor for over 90% of cases of allergic aspergillosis. Unequal to invasive aspergillosis, CPA emerges in non-immunocompromised patients, which debatably most ABPA patients are. The sickness of CPA is important involving weight loss, deeper fatigue, productive cough, major shortness of breath, and life-threatening hemoptysis.
Drug Class Segment Analysis
The antifungal segment is anticipated to hold around 60% share of the global aspergillosis treatment market by 2035. The dominance of antifungal drugs in the market can be accredited to their efficacy against infections and extensive implementation as a primary treatment choice. Healthcare providers' concentrate on developing modified and focused antifungal treatments is expected to maintain the segment's eminence, driving complete market growth. F2G, a key player in the aspergillosis treatment market, has accomplished a substantial milestone with the U.S. Food and Drug Administration's (FDA) approval of its New Drug Application (NDA) for Olorofim in December 2022, a revolutionary antifungal drug customized for invasive fungal infections, involving aspergillosis. This growth indicates a substantial development in antifungal treatments and presents promising aspects for patients combating life-threatening fungal infections in the market.
Our in-depth analysis of the global aspergillosis treatment market includes the following segments:
|
Type |
|
|
Drug Class |
|
|
Route of Administration |
|
|
Distribution Channels |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Aspergillosis Treatment Market Regional Analysis:
North American Market Insights
North America is expected to hold the largest share of the aspergillosis treatment market with almost 42% by 2035. North America will hold this position due to the reason that it has a high prevalence of situations that instigate the risk of aspergillosis, like lung diseases, HIV, and organ transplantation. This contributes to a wider patient pool needing HIV drug/medicine, and lung treatment. A calculated 7,199 deaths from fungal diseases happened in 2021 in North America. In North America, the most prevalent aspergillosis is the most clinically important section is Fumigati, comprising A. fumigatus, A. lentulus and A. udagawae among others.
European Market Insights
Europe is set to hold the second-largest share in the aspergillosis treatment market in the coming timeline globally because of the European Government’s rigorous initiatives to combat aspergillosis and respiratory infections. Moreover, on 25th of October 2023, WHO/Europe and the European Centre for Disease Prevention and Control (ECDC) announced the weekly European Respiratory Virus Surveillance Summary (ERVISS) an online platform that shows integrated surveillance information for aspergillosis, influenza, COVID-19 and RSV in the WHO European Region, involving the European Union/European Economic Area.
Aspergillosis Treatment Market Players:
- Bayer AG
- Company Overview
- Business Planning
- Main Product Offerings
- Financial Execution
- Main Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- WOT Analysis
- Mayne Pharma Group Limited
- PULMATRiX Inc.
- Endo International plc
- Johnson & Johnson Services Inc.
- GlaxoSmithKline plc
- Pfizer Inc.
- Abbott
- Takeda Pharmaceutical Company Limited
Recent Developments
- Endo International plc released of the first generic Noxafil (posaconazole) Injection marks a substantial growth in the aspergillosis treatment market. This generic posaconazole injection is particularly created to identify invasive aspergillosis in patients aged 13 and above, surrounding both adults and pediatric populations.Candida infections in clients who are at significant risk of creating these infections because of being seriously immunocompromised, like hematopoietic stem cell transplant (HSCT) payees with graft-versus-host disease (GVHD) or those with hematologic enmities with extended neutropenia (low white blood cell counts) from chemotherapy.
- Mayne Pharma Group Limited got approval from U.S.FDA for TolsuraTM (SUBA-Itraconazole Capsules). TOLSURA is a novel itraconazole development implemented to cure systemic fungal infections like aspergillosis, blastomycosis, and histoplasmosis. Tolsura is suggested for the therapy of blastomycosis (pulmonary and extrapulmonary), histoplasmosis (involving chronic cavitary pulmonary disease and disseminated non-meningeal histoplasmosis), and aspergillosis (pulmonary and extrapulmonary, in sufferers who are weak or who are stubborn to amphotericin B therapy).
- Report ID: 5410
- Published Date: Sep 11, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Aspergillosis Treatment Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




