Anti-counterfeit Pharmaceutical Packaging Market Outlook:
Anti-counterfeit Pharmaceutical Packaging Market size was valued at approximately USD 127.5 billion in 2025 and is projected to reach around USD 290.9 billion by the end of 2035, rising at a CAGR of approximately 8.6% during the forecast period 2026-2035. In 2026, the industry size of the Anti-counterfeit pharmaceutical packaging is estimated at USD 138.5 billion.

According to the Food and Drug Administration, drugs from the antibody and opioid classes are likely to have faced issues of unauthorized distribution. The government has brought new changes to its regulations and invested in elevating the anti-counterfeit packaging as an alternate solution to combat the global issue. This has created a mass demand for track-and-trace technology, tamper-evident seals, and many more. The expansion of sales for products like RFID tags and holographic films has taken an extraordinary growth.
Research and development in pharmaceutical packaging is amalgamated with regulatory policies, with innovation in the supply chain tracking. Most of the research and development emphasizes enhancing sterilization. Market players are even integrating blockchain and verification applications to lower the rate of counterfeiting. As the market demand is widely expanding, the research and development efforts have become interdisciplinary. Also, manufacturers are trying to ensure that anti-counterfeit technology is inculcated at the initial stage, which is also propelling the market growth.
Key Anti-counterfeit Pharmaceutical Packaging Market Insights Summary:
Regional Highlights:
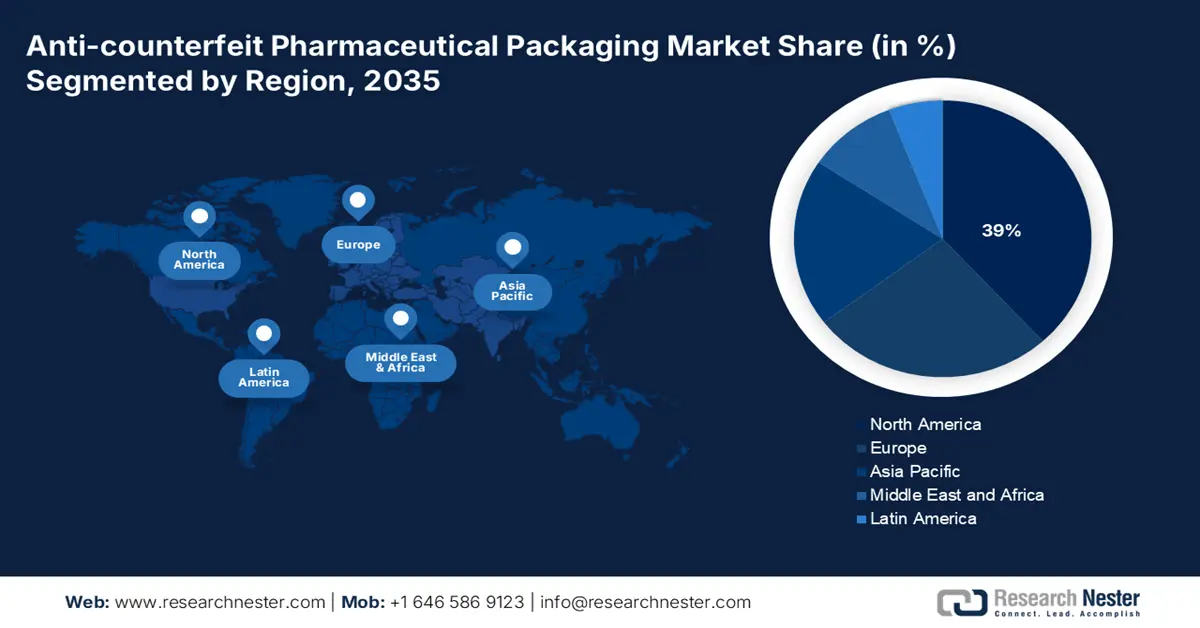
- By 2035, North America is projected to command a 39% share of the Anti-counterfeit Pharmaceutical Packaging Market, supported by increasing track-and-trace activity to reduce counterfeit incidents.
- Europe is anticipated to secure a growing share by 2035 as the fastest-growing region, underpinned by the rapid adoption of smart packaging solutions to eradicate counterfeit issues.
Segment Insights:
- The radio frequency identification segment is set to account for 34.5% of the market share by 2035 within the Anti-counterfeit Pharmaceutical Packaging Market, spurred by the rising requirement for the real-time monitoring of the drugs.
- The serialization segment is on track to attain a substantial market share by 2035, reinforced by stringent regulatory mandates.
Key Growth Trends:
- Regulatory mandates and government support
- Rising cases of counterfeit drugs
Major Challenges:
- Government pricing and barriers to reimbursement
- Barriers of Regulation delay
Key Players: Avery Dennison, SICPA, 3M, Zebra Technologies, Authentix, DuPont, CCL Industries, Uflex, Schreiner MediPharm, Arjo Solutions, Essentra, Amcor, Kurz, Samsung SDI, Huhtamaki.
Global Anti-counterfeit Pharmaceutical Packaging Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 127.5 billion
- 2026 Market Size: USD 138.5 billion
- Projected Market Size: USD 290.9 billion by 2035
- Growth Forecasts: 8.6% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (39% Share by 2035)
- Fastest Growing Region: Europe
- Dominating Countries: United States, China, Germany, Japan, United Kingdom
- Emerging Countries: India, Brazil, South Korea, Mexico, Indonesia
Last updated on : 9 September, 2025
Anti-counterfeit Pharmaceutical Packaging Market - Growth Drivers and Challenges
Growth Drivers
- Regulatory mandates and government support: Governments play a significant role within the global platform to reduce the risk of counterfeit products by strengthening the regulation of packaging. In Europe, the Falsified Medicines Directive needs safety features such as tamper-evident packaging and 2D barcodes on all medicines sold. Similarly, in the U.S., the FDA has implemented the Drug Supply Chain Security Act (DSCSA), which mandates that tracing from manufacturers to the dispenser is necessary using an interoperable electronic system. Various other countries are also enforcing or strengthening serialization mandates, primarily to curb the supply of counterfeit drugs into global supply chains.
- Rising cases of counterfeit drugs: According to the World Economic Forum in December 2024, at least 1 in 10 medicines in middle and low-income countries are falsified or substandard. Such counterfeit drugs usually contain incorrect dosages and harmful ingredients, failing to treat the condition of the patient and increasing antimicrobial resistance. With the rise in awareness, all the stakeholders are under immense pressure to secure the supply chain of the drugs. The burgeoning threat has compelled the pharmaceutical companies to invest in modern packaging solutions such as holograms, serialization, etc. The surge in counterfeit activities has also increased the incorporation of a strict policy framework, further augmenting the market growth.
- Technological advancement and smart packaging techniques: The inclusion of the RFID/NFC chips and QR codes is fostering the market forward. Such innovations are allowing transparency in the supply chain and real-time authentication. Also, the cost of these technologies is decreasing, and manufacturers are finding them viable for a broader range of pharmaceutical products. Also, the amalgamation of the IoT and AI packaging system upgrades the detection of anomalies and monitoring processes. These methods make the supply chain more robust against any attempts at counterfeiting. Also, in this way, pharmaceutical companies safeguard the reputation of their brands.
Challenges
- Government pricing and barriers to reimbursement: The cost of manufacturing Anti-counterfeit pharmaceutical packaging is higher compared to the traditional methods. Additionally, U.S. Medicaid coverage for the Anti-counterfeit pharmaceutical packaging solution is very less, creating a barrier for low-income consumers to access the drugs. Also, a reduction in EU price caps for the manufacturer's margin crippled the profit margin rate for the companies, and negotiations on the bulk deals were conducted.
- Barriers of Regulation delay: The complex and frequent changes in the regulatory standards for packaging have created a delay in the process. A complex issue is addressed by global businesses as per the differentiation of compliance between U.S. and EU regulations. This creates a barrier for the business to easily penetrate the market and launch its new product to gain higher revenue.
Anti-counterfeit Pharmaceutical Packaging Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
8.6% |
|
Base Year Market Size (2025) |
USD 127.5 billion |
|
Forecast Year Market Size (2035) |
USD 290.9 billion |
|
Regional Scope |
|
Anti-counterfeit Pharmaceutical Packaging Market Segmentation:
Technology Segment Analysis
The radio frequency identification is rising as the most dominant technology and is projected to garner 34.5% of the market share by 2035. The market growth can be attributed to the rising requirement for the real-time monitoring of the drugs. RFID tags are different from conventional tags and can be read wirelessly across various units. These features make RFID tags highly efficient. Also, the segment is gaining traction owing to its ability to seamlessly serialize. Governments across the world are investing to accelerate the deployment of RFID systems. Additionally, technological advancements have remarkably reduced the cost of these tags, making them commercially viable to use.
Application Segment Analysis
The serialization segment is anticipated to witness significant growth and is expected to garner a significant market share by 2035. The push for serialization has been propelled by the stringent regulatory mandates. In various European Nations, laws require prescription medicines to carry unique tamper-evident features. These types of mandates have compelled pharmaceutical companies to include serialization systems, further bolstering the expansion of the market. Additionally, serialization renders significant security benefits by inculcating end-to-end traceability methods. These technological advancements transform serialization into a strategic advantage, making it a prominent growth driver.
Our in-depth analysis of the Anti-counterfeit pharmaceutical packaging market includes the following segments:
|
Segment |
Sub Segments |
|
Technology |
|
|
Application |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Anti-counterfeit Pharmaceutical Packaging Market - Regional Analysis
North America Market Insights
North America holds the dominant leadership within the market of Anti-counterfeit pharmaceutical packaging, with a global market share of 39%. Increasing the track-and-trace activity to reduce counterfeit incidents, the market is witnessing a spontaneous growth in the region. Latest technologies such as blockchain and Yammer evident seals are expected to be the most revenue-generating segments within the market. The U.S. market has achieved scalability with the increasing number of counterfeit activities. Medicare spending has created better opportunities for accessibility in the market.
The Anti-counterfeit pharmaceutical packaging market of Canada is projected to acquire a significant market share within North America. The government has fostered the growth of the safe packaging market through allocating federal spending on healthcare. Technological advancement and higher scope of research and development have been the driving factors for the development of the market. In 2024, the Royal Canadian Mounted Police confiscated more than 1 million counterfeit pills during an operation. Also, the country is strengthening its pharmaceutical regulations to align with international standards.
Europe Market Insights
The Anti-counterfeit pharmaceutical packaging in Europe is the fastest-growing region. In February 2024, Europol conducted a high-impact investigation operation called SHIELD IV, with support from the EU Intellectual Property Office, and authorities seized USD 70 million of fake drugs. Companies and pharmaceutical firms are adopting smart packaging solutions to eradicate such issues. Also, robust research and development in the region is fueling the market growth. Such trends are compelling more companies to adopt anti-counterfeit packaging to secure trust in e-commerce channels.
In Germany growth of Anti-counterfeit pharmaceutical packaging has secured a dominant position. The regulatory authorities have given importance to patient safety and encourage widespread implementation of serialization systems. Germany is a leading country in the market and is integrating blockchain technology into the pharmaceutical industry. These technological integrations are working tirelessly to make the most trusted and robust drug supply chains in the country. The leadership of the country is reinforced by the active participation in initiatives associated with curbing falsified medicines.
APAC Market Insights
The APAC market is also projected to garner a significant revenue share by 2035, owing to the high prevalence of counterfeit drugs in the region. Also, the region is witnessing the rapid expansion of the pharmaceutical industry, thereby accelerating the adoption across the APAC market. The growth of the market is also propelled by the growth of online pharmacies and cross-border e-commerce. Pharmaceutical companies in the region are responding promptly by including innovative packaging solutions that render an explicit indication of authenticity, thereby increasing patient trust and reinforcing brand protection.
In India, the data published by the Indian Journal of Pharmacy Practice in June 2025, between 12- 24% of all pharmaceuticals supplied in the country are found to be fake. Various non-profit organizations are providing industry standards identification systems by developing a “track and trace” solution, such as DataKart Trace. The advent of the Traceability and Authentication Forum brought together policymakers and experts to strengthen efforts for anti-counterfeiting laws. The country is enforcing the defense against counterfeit medicines, and these efforts endeavor to protect the country’s reputation.
Key Anti-counterfeit Pharmaceutical Packaging Market Players:
- Avery Dennison
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- SICPA
- 3M
- Zebra Technologies
- Authentix
- DuPont
- CCL Industries
- Uflex
- Schreiner MediPharm
- Arjo Solutions
- Essentra
- Amcor
- Kurz
- Samsung SDI
- Huhtamaki
The global market of Anti-counterfeit pharmaceutical packaging has been dominated by five main players that possess a market share of 48%. Avery Dennison, SICPA, and 3M are the top-rated three companies that have incorporated technological innovation the escalate the business performance. RFID and blockchain have been relied on for the business expansion of Avery Dennison and SICPA. Whereas the escalation of tamper-evident as well as track-and-trace solutions has been fostered by Zebra Technology and 3 M.
Here is a list of key players operating in the global market:
Recent Developments
- In January 2025, Systech International introduced its AI-powered serialization and track-and-trace solution for global pharmaceutical supply chains. This platform delivers AI-powered authentication tailored to the life sciences and pharmaceutical sectors. It’s engineered to safeguard packaging quality, protect patient safety, and preserve brand integrity.
- In January 2025, Vina CHG rolled out Vinacheck smart software, enabling real-time product authentication, tracking, and customer engagement—integrated into Anti-counterfeit pharmaceutical packaging.
- Report ID: 3043
- Published Date: Sep 09, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Anti-counterfeit Pharmaceutical Packaging Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




