IV Fluid Monitoring Devices Market Outlook:
IV Fluid Monitoring Devices Market size was over USD 5.12 billion in 2025 and is poised to exceed USD 10.36 billion by 2035, growing at over 7.3% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of IV fluid monitoring devices is estimated at USD 5.46 billion.
The major element to influences the market expansion is the growing prevalence of hospital-acquired infection. In 2022, it was estimated by WHO that, at least one healthcare-associated infection (HAI) will be acquired by seven patients in high-income nations and fifteen patients in low- and middle-income countries out of every 100 patients in acute-care hospitals during their hospital stay. Approximately 10% of impacted patients will pass away due to their HAI. Hence, the market revenue for IV fluid monitoring devices is set to increase in order to reduce hospital acquired infection.
Additionally, the majority of pharmaceutical and medical device companies have focused on integrating cutting-edge technologies into infusion systems in order to enhance the efficacy, safety, and efficiency of drugs while decreasing the possibility of unfavorable reactions brought on by an overdose or underdose. Therefore, this factor is also set to drive the IV fluid monitoring devices market expansion.
Key IV Fluid Monitoring Devices Market Insights Summary:
Regional Highlights:
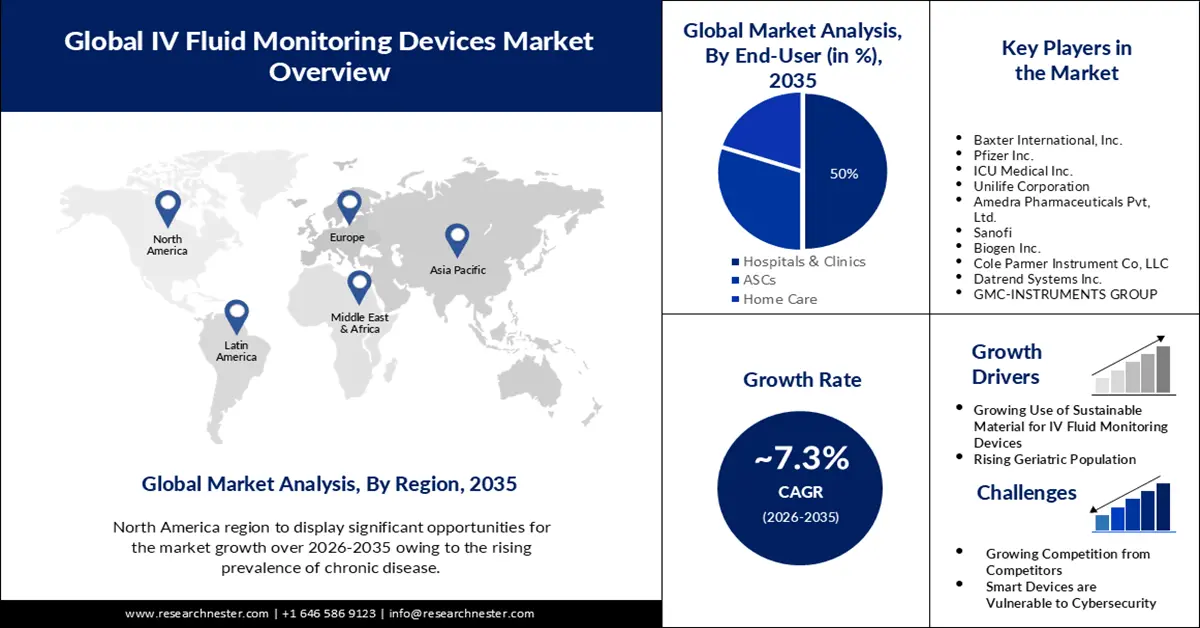
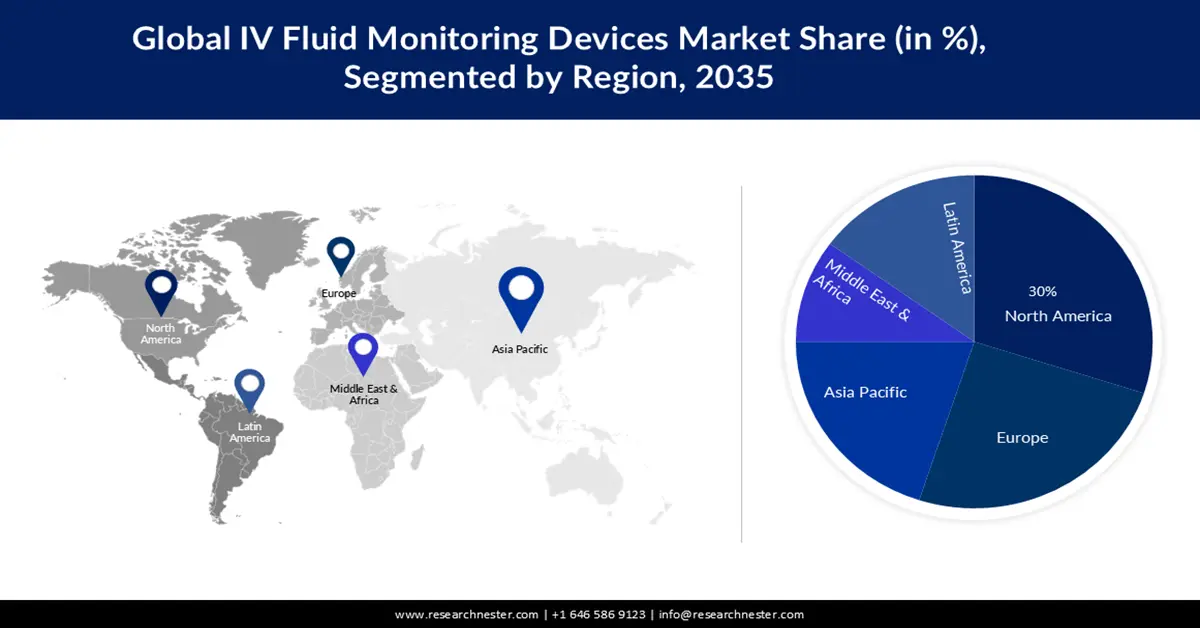
- In the IV fluid monitoring devices market, North America is expected to command a 30% share by 2035, supported by escalating chronic disease prevalence and the rising integration of smart medical robotics that minimize medication errors.
- Europe is poised to secure a substantial share by 2035, underpinned by the region’s shift toward integrated care models and wider adoption of home-care infusion systems with remote programming capabilities.
Segment Insights:
- By 2035, the hospitals & clinics segment in the IV fluid monitoring devices market is projected to hold over 50% share, reinforced by increasing patient volumes and expanding partnerships between multispecialty hospitals and clinic networks.
- The desktop segment is anticipated to capture about 60% revenue share by 2035, attributed to its superior reliability, long-term performance, and expanding investments in advanced desktop-based monitoring systems.
Key Growth Trends:
- Growing Use of Sustainable Material for IV Fluid Monitoring Devices
- Rising Geriatric Population
Major Challenges:
- Growing Competition from Competitors
- Smart Devices are Vulnerable to Cybersecurity
Key Players: Baxter International Inc., Pfizer Inc., ICU Medical Inc., Unilife Corporation, Amedra Pharmaceuticals Pvt. Ltd., Sanofi, Biogen Inc., Cole Parmer Instrument Co. LLC, Datrend Systems Inc., GMC-INSTRUMENTS GROUP.
Global IV Fluid Monitoring Devices Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 5.12 billion
- 2026 Market Size: USD 5.46 billion
- Projected Market Size: USD 10.36 billion by 2035
- Growth Forecasts: 7.3%
Key Regional Dynamics:
- Largest Region: North America (30% share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, China, Germany, India, Japan
- Emerging Countries: China, India, Brazil, Mexico, Turkey
Last updated on : 27 November, 2025
IV Fluid Monitoring Devices Market - Growth Drivers and Challenges
Growth Drivers
-
Growing Use of Sustainable Material for IV Fluid Monitoring Devices - With the development of biodegradable materials, there currently exists a great chance to revolutionize healthcare technologies through the implementation of sensors that will naturally break down after use. Implantable electronic devices composed of these materials reduce chronic inflammatory reactions, do not require extraction or reoperation, and so present appealing possibilities for biomedical technology in the future. Since they produce less electronic or medical waste and, consequently, have a lower carbon footprint, eco-friendly sensor systems made of biodegradable materials may also help alleviate some of the most pressing environmental problems. As a result, the market is poised to rise for IV fluid monitoring devices.
- Rising Geriatric Population - As per the WHO prediction, the number of human beings in the world who are 60 years of age or older is expected to double (to 2.1 billion) by 2050. It is poised that between the year 2020 and 2050, the number of people 80 years of age or older is set to treble, reaching 426 million. Intravenous (IV) fluid therapy is often required for adult hospital inpatients in order to avoid or treat issues related to their fluid and/or electrolyte status. It may be challenging and complex to determine the right IV fluid dosage, mix, and rate of administration. Careful consideration of each patient's unique demands must guide these decisions.
- Surge in Importance of Telemedicine in Monitoring - Covid19 proved the importance of telemedicine. Through remote consultation, continuous monitoring, and patient education through phone and videoconferencing in the context of the coronavirus disease, technology has been extremely helpful in maintaining patient continuity. Telemedicine systems will continue employing IV fluid transfer devices. Patients are experiencing accessibility and convenience as essential components of healthcare delivery as a result of the development of remote monitoring and consultations. Therefore, the IV fluid monitoring devices market demand is projected to surge over the coming years.
Challenges
-
Growing Competition from Competitors - There is a lot of competition in the market as there are many local and international suppliers. Prominent international vendors face fierce competition from tiny regional vendors who provide comparable products at comparatively lower prices, notwithstanding their extensive distribution networks and contracts with top hospitals and healthcare facilities. Customers may additionally choose to buy premium goods at discounted costs. The marketplace is price-sensitive and volume-driven. There is fierce rivalry among suppliers, which is resulting in price wars. In an effort to remain competitive in the IV fluid monitoring devices market, vendors are concentrating on lowering the cost of their products in order to increase sales volume. The top vendors are right now operating on thin profit margins. Consequently, over the course of the forecast period, the pricing pressure is anticipated to have an impact on the IV fluid monitoring devices market expansion for intravenous (IV) fluid monitoring devices.
- Smart Devices are Vulnerable to Cybersecurity
- Rising Adoption of Oral Drugs
IV Fluid Monitoring Devices Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
7.3% |
|
Base Year Market Size (2025) |
USD 5.12 billion |
|
Forecast Year Market Size (2035) |
USD 10.36 billion |
|
Regional Scope |
|
IV Fluid Monitoring Devices Market Segmentation:
End-User Segment Analysis
The hospitals & clinics segment in the IV fluid monitoring devices market is set to gather the highest share of over 50% during the forecast period. Due to the availability of better patient care, convenient appointment rescheduling, and beneficial reimbursement scenarios, a large number of persons in this category are receiving treatment in clinics. Clinic visits from patients are on the rise. Moreover, for instance, In the United States, there were over 1,999 mobile clinics operating in 2021, providing care for an estimated over 6 million at-risk people annually. Hospitals that work with clinics have so evolved into hospital-based healthcare providers. Compared with clinics, hospitals are reimbursed less. Hence, there has been growing collaboration between hospitals & clinics. Furthermore, large hospitals, including hospital groups and multispecialty hospitals, are either privately owned or sponsored by the government. These hospitals can accommodate patients within a region since they have a larger number of hospital beds.
Type Segment Analysis
IV fluid monitoring devices market from the desktop segment is set to gather the highest revenue share of about 60% by 2035. This is because desktop IV fluid monitoring devices could be used to carry out huge tasks as compared to portable devices. Desktop drives are better suited for large-scale, professional applications because they put lifespan, security, and dependability above portability. Furthermore, even though portable devices are making their space in the market, people’s preference for desktop-based devices is high due to the amount of reliability they provide. Furthermore, investment has been made in improving the desktop system which is further attracting more revenue in the market.
Our in-depth analysis of the global IV fluid monitoring devices market includes the following segments:
|
End-User |
|
|
Type |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
IV Fluid Monitoring Devices Market - Regional Analysis
North American Market Insights
The North America industry is poised to dominate majority revenue share of 30% by 2035. This growth of the market in this region is set to be dominated by rising prevalence of chronic disease. One or more chronic illnesses, such as diabetes, cancer, heart disease, or stroke, affect six out of ten Americans. Furthermore, the technology is in this region is getting smarter. This is since, the biggest issues in hospitals are medication mistakes. Numerous guidelines and reports are created for numerous organizations and governments in an effort to prevent drug mishaps. As a result, the adoption of medical robotics is growing in this segment. By decreasing the amount of time, they need to spend preparing drugs, the new robotic technology enables nurses and chemists to spend their time more productively. Since incorrect dose applications can be followed up with the right preparation of the dose, the created communication server prevents leakage losses that result in financial losses.
European Market Insights
The IV fluid monitoring devices market in Europe is also projected to have notable growth over the coming years. Most Europeans place a high value on their health and anticipate receiving quality medical treatment. Moreover, integrated models, in which hospitals collaborate closely with primary care, community care, and home care, have already replaced the conventional hospital-centric and doctor-centric patterns of care in the majority of health systems in this region. Hence, further long hospital stays and significant healthcare costs are avoided when managing chronic diseases with home infusion therapy devices, that offer safe, precise, and precise drug delivery based on the patient's demands. The IV fluid monitoring devices market is expanding as a result of the increasing number of home-care infusion pumps having wireless and remote programming capabilities. These characteristics enable chemists and clinicians to monitor and establish pharmaceutical dose settings and reduce the frequency of hospital visits.
IV Fluid Monitoring Devices Market Players:
- Baxter International, Inc.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Pfizer Inc.
- ICU Medical Inc.
- Unilife Corporation
- Amedra Pharmaceuticals Pvt, Ltd.
- Sanofi
- Biogen Inc.
- Cole Parmer Instrument Co, LLC
- Datrend Systems Inc.
- GMC-INSTRUMENTS GROUP
Recent Developments
- A group of University of Washington undergraduates studying electrical and computer engineering (ECE) have created a low-cost intravenous fluid flow monitoring device that can be used in low- and middle-income nations. Dr. Gregory Valentine of the UW School of Medicine's Department of Paediatrics served as the team's leader.
- Leading innovator in drug delivery technology, Baxter International Inc., today released the findings of the Fluid Response Evaluation in Sepsis Hypotension and Shock (FRESH) study conducted with the Starling Fluid Management Monitoring System, which was just published in CHEST. Using a non-invasive dynamic assessment to guide intravenous (IV) fluid and vasopressor administration reduces net fluid balance, mechanical ventilation, and kidney injury in patients with septic shock, according to the results of the FRESH study, which was conducted at 13 hospitals in the United States and the United Kingdom.
- Report ID: 5528
- Published Date: Nov 27, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
IV Fluid Monitoring Devices Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




