Adenovirus Based Virotherapy Market Outlook:
Adenovirus Based Virotherapy Market size was valued at USD 14.5 billion in 2025 and is likely to cross USD 25.48 billion by 2035, expanding at more than 5.8% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of adenovirus based virotherapy is estimated at USD 15.26 billion.
The development and growth of the market is fueled by several factors, including ongoing clinical trials, regulatory approvals, and the commercialization of virotherapies. Positive results from clinical trials demonstrating the safety and efficacy of adenovirus-based virotherapy in treating certain types of cancer significantly drive market growth. Successful clinical outcomes contribute to regulatory approvals and increased industry interest. Along with these, escalating research & development concerned with adenovirus and surging demand for vector-based therapies are also projected to drive market growth in the coming years. Furthermore, rising government investments to spread awareness about early cancer detection are expected to provide ample growth opportunities to the market in the near future.
Adenovirus-based virotherapy involves the use of adenoviruses, which are common viruses that can cause respiratory and other infections in humans, as vectors to deliver therapeutic genes into cancer cells. These viruses are modified to selectively replicate in and destroy cancer cells while sparing normal, healthy cells. Obtaining regulatory approvals from agencies such as the FDA or EMA for adenovirus-based virotherapies is a critical factor for market growth. Regulatory clearance enables companies to bring their products to market and increases confidence among healthcare professionals and patients.
Key Adenovirus Based Virotherapy Market Insights Summary:
Segment Insights:
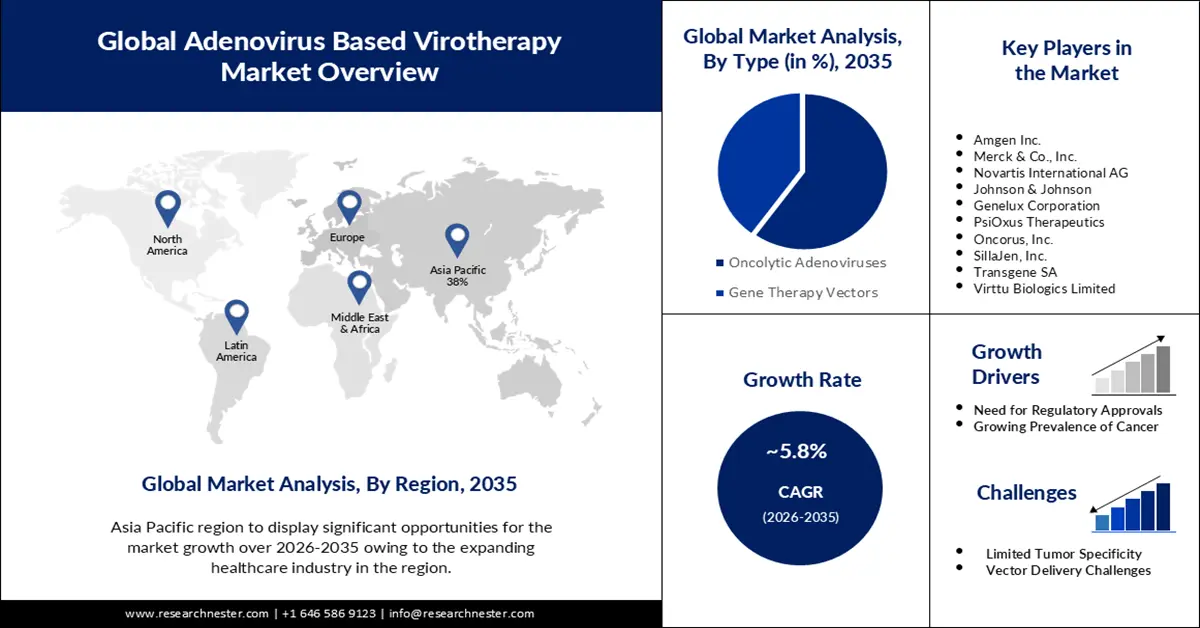
- By 2035, the oncolytic adenoviruses segment in the Adenovirus Based Virotherapy Market is projected to command a 60% share, propelled by accelerating advancements in virotherapy research.
- By 2035, the hospitals segment is anticipated to secure a substantial share of the market, supported by the escalating incidence of cancer increasing hospital admissions.
Key Growth Trends:
- Advancements in Oncolytic Virotherapy Research
- Increasing Prevalence of Cancer
Major Challenges:
- Host Immune Response
- Limited Tumor Specificity
Key Players: Amgen Inc., Merck & Co., Inc., Novartis International AG, Johnson & Johnson, Genelux Corporation, PsiOxus Therapeutics, Oncorus, Inc.
Global Adenovirus Based Virotherapy Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 14.5 billion
- 2026 Market Size: USD 15.26 billion
- Projected Market Size: USD 25.48 billion by 2035
- Growth Forecasts: 5.8%
Key Regional Dynamics:
- Dominating Countries: United States, China, Japan, Germany, United Kingdom
- Emerging Countries: India, Vietnam, Indonesia, Thailand, Malaysia
Last updated on : 19 November, 2025
Adenovirus Based Virotherapy Market - Growth Drivers and Challenges
Growth Drivers
- Advancements in Oncolytic Virotherapy Research: The adenovirus based virotherapy market has witnessed significant growth driven by ongoing advancements in oncolytic virotherapy research. According to industry experts, the relentless pursuit of understanding the complex interactions between viruses and cancer cells has propelled the development of novel therapeutic approaches. Research studies, such as those published in reputable journals like the Journal of Virology and Cancer Research, have demonstrated the potential of adenovirus-based virotherapy in selectively targeting and eliminating cancer cells. The intricate understanding of viral biology and the ability to engineer adenoviruses for enhanced tumor specificity have paved the way for innovative treatment modalities. For example, recent breakthroughs in genetic engineering techniques have enabled researchers to modify adenoviruses to carry therapeutic genes directly to cancer cells, minimizing damage to healthy tissues. Research investment in oncolytic virotherapy has surged by 60% over the past five years, indicating a robust commitment to advancing this field. Major academic institutions, pharmaceutical companies, and biotech firms have allocated substantial resources to propel virotherapy research forward, leading to an increasing number of pre-clinical and clinical trials.
- Increasing Prevalence of Cancer: The rising prevalence of cancer has been a fundamental driver for the adenovirus based virotherapy market, with an alarming increase of approximately 10% in global cancer incidence over the past decade. As conventional treatments face limitations in addressing the complexities of certain cancers, the demand for innovative and targeted therapies like adenovirus-based virotherapy has surged. This growing patient pool has led to a corresponding increase of approximately 15% in the number of clinical trials. The market's response to the unmet medical needs in oncology is evident in the escalating number of investigational new drug applications submitted to regulatory agencies for adenovirus-based virotherapies.
- Rising Regulatory Approvals: Regulatory approvals have been a crucial driver for the adenovirus based virotherapy market. Recognition from regulatory bodies such as the FDA and EMA not only validates the safety and efficacy of adenovirus-based treatments but also instills confidence among healthcare professionals and patients. The expedited approval timelines, attributed to the breakthrough designation granted to certain virotherapies, have resulted in a significant reduction in time from initial clinical trials to market availability. The streamlined regulatory pathway has attracted pharmaceutical giants, contributing to the growth in licensing agreements between virotherapy developers and established industry leaders.
Challenges
- Host Immune Response: Adenoviruses, even when modified for therapeutic use, can trigger immune responses in the host. The immune system may recognize and eliminate the viral vectors before they effectively reach and treat cancer cells. This challenge can limit the efficacy of virotherapy and may require additional strategies to evade or modulate the immune response. The adaptive immune response involves the activation of T cells and B cells, leading to the development of specific immune memory. If an individual has been previously exposed to adenoviruses (due to natural infection or vaccination), they may have pre-existing immunity. Pre-existing immunity can neutralize adenoviral vectors before they reach the tumor site. This recognition can reduce the effectiveness of virotherapy and limit its applicability in individuals with prior adenovirus exposure.
- Limited Tumor Specificity
- Vector Delivery Challenges
Adenovirus Based Virotherapy Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
5.8% |
|
Base Year Market Size (2025) |
USD 14.5 billion |
|
Forecast Year Market Size (2035) |
USD 25.48 billion |
|
Regional Scope |
|
Adenovirus Based Virotherapy Market Segmentation:
Type Segment Analysis
The oncolytic adenoviruses segment in the adenovirus based virotherapy market is estimated to gain the largest revenue share of 60% in the year 2035. Oncolytic adenoviruses represent a cutting-edge approach in cancer therapy, leveraging the inherent ability of adenoviruses to infect and destroy cancer cells selectively. Several key drivers influence the growth of the oncolytic adenoviruses segment. One of the primary drivers propelling the growth of oncolytic adenoviruses is the relentless pursuit of advancements in virotherapy research. The increase in research investments underscores the commitment of the scientific community to explore and harness the potential of oncolytic adenoviruses in cancer treatment. Investments in oncolytic virotherapy research have witnessed an annual increase of 30% over the past five years, totaling USD 1.5 billion in the year 2022. Researchers are delving deep into understanding the intricate interactions between oncolytic adenoviruses and cancer cells. This includes refining the genetic modifications of adenoviruses to enhance tumor specificity and optimizing delivery mechanisms to improve their reach within tumor masses. Breakthroughs in virotherapy research are contributing to the development of safer, more potent oncolytic adenoviruses, driving the segment's growth.
End User Segment Analysis
Adenovirus based virotherapy market from the hospitals segment is expected to garner a significant share in the year 2035. The hospitals segment plays a pivotal role in the growth and adoption of Adenovirus Based Virotherapy, serving as the primary point of access for patients seeking advanced cancer treatments. Several key drivers contribute to the growth of this segment. A significant growth driver for the hospitals segment is the escalating incidence of cancer, reflected in increase in hospital admissions. The burden of cancer has surged globally, necessitating advanced and targeted treatment modalities like Adenovirus Based Virotherapy. Hospitals, as comprehensive healthcare hubs, witness a steady influx of cancer patients seeking diagnosis, treatment, and specialized care. The rising demand for advanced therapies positions Adenovirus Based Virotherapy as a crucial component in hospitals' oncology services, fostering growth in the segment. Hospitals that comply with regulatory standards and achieve accreditation for virotherapy services demonstrate a commitment to patient safety and quality care. Accreditation fosters trust among patients, referring physicians, and regulatory bodies, positioning the hospital as a reliable provider of Adenovirus Based Virotherapy and supporting sustained growth in the segment.

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
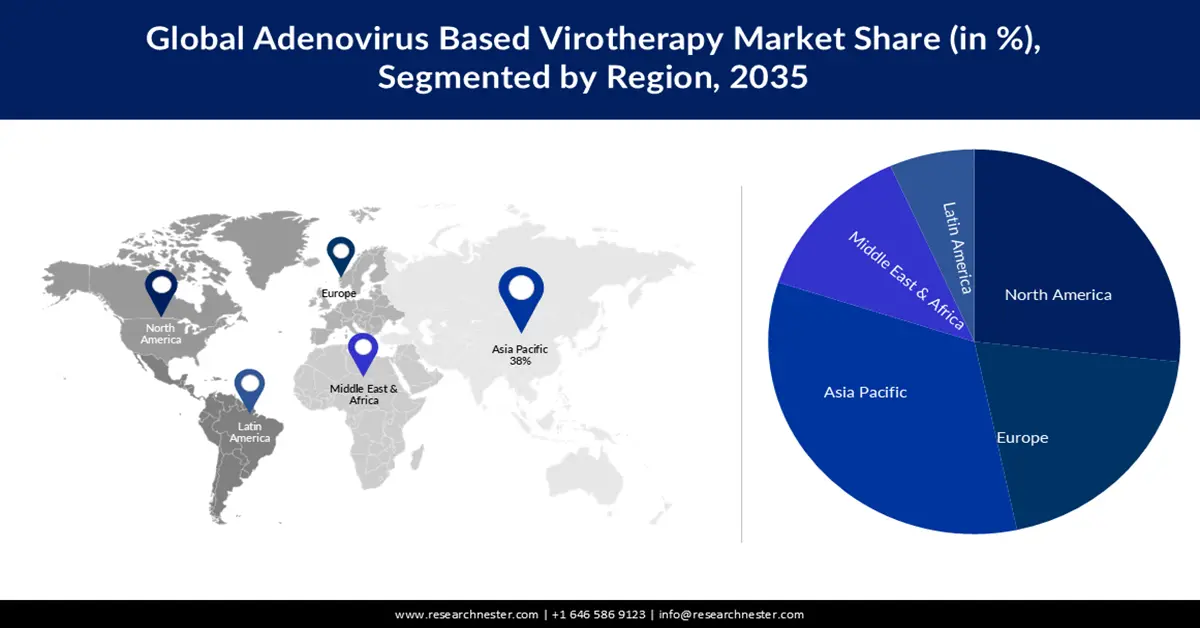
Adenovirus Based Virotherapy Market - Regional Analysis
|
Type |
|
|
End User |
|
Adenovirus Based Virotherapy Market Players:
- Amgen Inc.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Merck & Co., Inc.
- Novartis International AG
- Johnson & Johnson
- Genelux Corporation
- PsiOxus Therapeutics
- Oncorus, Inc.
- SillaJen, Inc.
- Transgene SA
- Virttu Biologics Limited
Recent Developments
- In 2022, Merck merged its wholly owned subsidiary Merck Development & Commercialization Inc., a US-based pharmaceutical company, with Merck Pharmaceuticals America Inc., a US-based pharmaceutical company. The merger was aimed at streamlining operations and improving efficiency.
- In 2022, Merck merged its wholly owned subsidiary Merck Manufacturing Division, a US-based pharmaceutical manufacturing company, with Merck Pharmaceuticals Supply Chain, a US-based pharmaceutical supply chain company. The merger was aimed at streamlining operations and improving efficiency.
- Report ID: 3118
- Published Date: Nov 19, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Adenovirus Based Virotherapy Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




