Long-acting Beta-agonists Market Outlook:
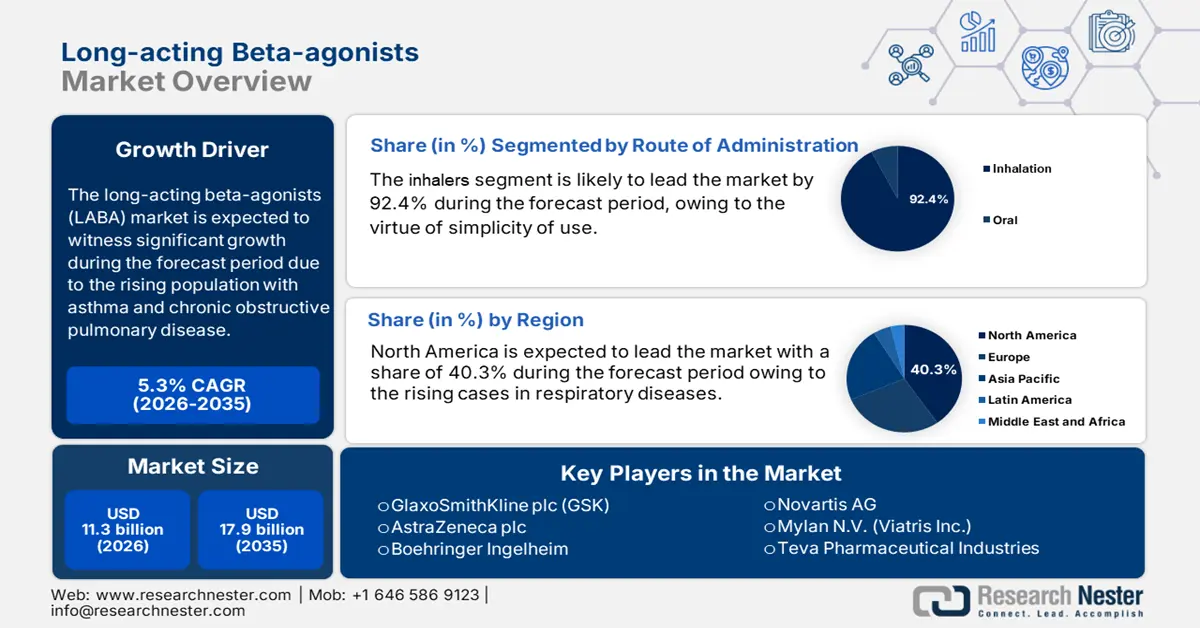
Long-acting Beta-agonists Market size was USD 11.3 billion in 2025 and is anticipated to reach USD 17.9 billion by the end of 2035, increasing at a CAGR of 5.3% during the forecast period, i.e., 2026-2035. In 2026, the industry size of long-acting beta-agonists is evaluated at USD 11.9 billion.
The international demand for the market is effectively fueled by a rise in population across different nations, who are readily being affected by chronic obstructive pulmonary disease as well as asthma. According to an article published by America Lung Association in July 2024, an estimated 3,517 people died from asthma, which is positively impacting the market’s development. Besides, there has been a decrease in the death rate from 1.7 per 100,000 population to 1.0 over the past four years. In addition, 59% of asthma-based deaths were prevalent among women, and the death rate was 27% higher among women in comparison to the male population.
Furthermore, as per an article published by the World Health Organization (WHO) in November 2024, chronic obstructive pulmonary disease (COPD) is considered the fourth leading cause of death, resulting in 3.5 million deaths, which is 5% of the overall deaths. In addition, almost 90% of COPD deaths take place within 70 years of age, particularly across low- and middle-income countries (LMIC), which is also uplifting the long-acting beta-agonists (LABA) market. Besides, tobacco smoking caters to more than 70% of COPD cases in developed countries, while in developing nations, it accounts for 30% to 40% of COPD cases, thereby making it suitable for bolstering the overall market.
Key Long-acting Beta-agonists Market Insights Summary:
Regional Insights:
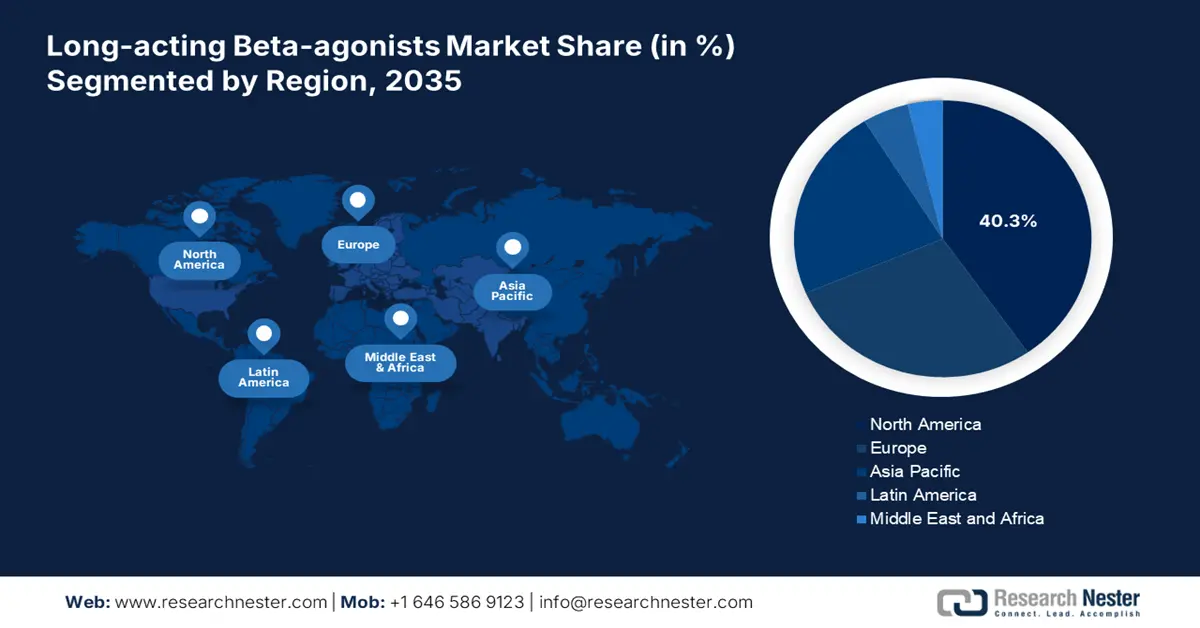
- North America in the Long-acting Beta-agonists Market is anticipated to command a 40.3% share by 2035, underpinned by escalating respiratory disorder prevalence and strengthened federal investment for chronic illness management.
- Asia Pacific is set to represent the fastest-growing regional share through 2035, supported by expanding healthcare infrastructure and increasing adoption of fixed-dose LABA/ICS combinations.
Segment Insights:
- The Inhalation segment in the Long-acting Beta-agonists Market is expected to hold a dominant 92.4% share by 2035, propelled by its ability to deliver LABAs directly to the lungs for faster onset of action at lower dosages with minimized systemic effects.
- The Combination Therapies (LABA/ICS) segment is projected to secure the second-largest share by 2035, stimulated by its clinical effectiveness in reducing severe asthma risk across varying patient severity levels.
Key Growth Trends:
- An increase in Medicare and Medicaid programs
- Proven hospitalization reduction and cost efficiency
Major Challenges:
- Pricing limitations in government-based healthcare systems
- Patient cost-effectiveness and out-of-pocket expenses
Key Players: GlaxoSmithKline plc (GSK), AstraZeneca plc, Boehringer Ingelheim, Novartis AG, Mylan N.V. (Viatris Inc.), Teva Pharmaceutical Industries, Chiesi Farmaceutici S.p.A., Cipla Ltd., Sun Pharmaceutical Industries Ltd., Astellas Pharma Inc., Takeda Pharmaceutical Company, Zambon S.p.A., Orion Corporation, Glenmark Pharmaceuticals, SK Bioscience Co., Ltd., Samsung Biologics, Lupin Limited, CSL Limited (Seqirus), Bio-pharma Sdn Bhd, Neopharma.
Global Long-acting Beta-agonists Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 11.3 billion
- 2026 Market Size: USD 11.9 billion
- Projected Market Size: USD 17.9 billion by 2035
- Growth Forecasts: 5.3% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (40.3% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, China, Germany, Japan, United Kingdom
- Emerging Countries: India, Brazil, South Korea, Indonesia, Mexico
Last updated on : 30 September, 2025
Long-acting Beta-agonists Market - Growth Drivers and Challenges
Growth Drivers
- An increase in Medicare and Medicaid programs: The aspect of Medicare Part D spending, government expenditure on healthcare, including generic and branded drug combinations such as fluticasone/salmeterol and budesonide/formoterol, which positively impacts the market. According to an article published by NLM in October 2024, Medicare Part D accounts for USD 13.3 billion, and there has also been an increase in insulin prices by 250%. Besides, the Inflation Reduction Act (IRA) has imposed penalties, leading to a surge in prices, with a USD 35 per month cap.
- Proven hospitalization reduction and cost efficiency: This has a great influence on the market, resulting in the early application of LABA drugs for patients suffering from chronic respiratory diseases. As stated in the February 2025 NLM article, the ICS/LABA prescription in Canada caters to 37.4% of its population, along with 48.0% for ICS/LABA and SABA maintenance and 56.2% in the U.S. Meanwhile, in China, the ICS/LABA as maintenance accounts for 59.5% of patients, while 70.7% in Japan, thereby suitable for uplifting the overall market internationally.
- Expansion in healthcare accessibility: This is extremely essential for the long-acting beta-agonists (LABA) market since it readily promotes public health, enables early disease management, and diminishes premature disability and deaths. As per the June 2024 ASPE Government article, the national uninsured rate in America has reached 7.7%, while marketplaces have successfully expanded insurance coverage from 12 million to more than 21 million, denoting an increase of over 77%. Besides, there has been a surge in people’s enrollment in the ACA Medicaid by almost 50% between 2020 and 2024, accounting for 15.5 million to 23.2 million, thus creating an optimistic outlook for the overall market.
Asthma Death Rates Among Different Individual Categories (2024)
|
Individual Categories |
Male |
Female |
|
Total |
0.8% |
1.1% |
|
White |
0.6% |
0.9% |
|
Black |
2.4% |
2.4% |
|
Asia/Pacific Islander |
0.8% |
0.6% |
|
Latino |
0.6% |
0.8% |
Source: America Lung Association
Challenges
- Pricing limitations in government-based healthcare systems: The government in Europe has made stringent price caps on LABA drugs through reference pricing systems and centralized negotiations that have reshaped flexibility in manufacturers’ pricing. In 2023, different organizations have addressed these gaps by numerous approaches, including tactical collaborations with national health agencies in France and Italy to achieve comprehensive formulator inclusion. This sudden transition has permitted manufacturers to combat the reimbursement restrictions and effectively maintain a competitive position, thus suitable for the market.
- Patient cost-effectiveness and out-of-pocket expenses: In markets such as the U.S., co-insurance and high deductibles place a huge financial burden on patients, resulting in non-adherence and prescription abandonment. Besides, even if a LABA is covered on an insurance formulary, it might be placed on an increased cost-sharing tier. This has effectively limited the product’s uptake since patients are unable to afford their respective portion of the expense, thereby causing a hindrance in the market. However, manufacturers utilize copay assistance programs to overcome this barrier, but these are frequently unavailable to Medicaid or Medicare patients, leading to a significant accessibility barrier for vulnerable populations.
Long-acting Beta-agonists Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
5.3% |
|
Base Year Market Size (2025) |
USD 11.3 billion |
|
Forecast Year Market Size (2035) |
USD 17.9 billion |
|
Regional Scope |
|
Long-acting Beta-agonists Market Segmentation:
Route of Administration Segment Analysis
Inhalation segment in the long-acting beta-agonists market is anticipated to garner the largest share of 92.4% by the end of 2035. The segment’s upliftment is highly attributed to the aspect of delivering LABAs directly to the lungs, thereby ensuring an increase in the onset of action at a low dosage, while reducing systemic side effects. This is considered a targeted approach, which is the international treatment for administering obstructive airway diseases, such as COPD and asthma. The segment’s growth is further propelled by ongoing advancements in inhaler device technology, which boosts patient adherence and drug delivery efficacy, thus denoting an optimistic outlook for the segment.
Drug Type Segment Analysis
Combination therapies (LABA/ICS) segment in the market is projected to cater to the second-largest share during the forecast duration. The segment’s growth is highly fueled by its clinical advantage to successfully aid patients with mild, moderate, and critical persistent asthma. According to an article published by Heliyon in June 2024, a clinical study was conducted on 78,171 patients, wherein the ICS/LABA treatment as a single relief and maintenance therapy has been proven to be suitable to diminish the future risk of severe asthma by an estimated 60%, thereby suitable for the segment’s exposure.
Application Segment Analysis
Chronic obstructive pulmonary disease (COPD) segment in the long-acting beta-agonists (LABA) market is expected to constitute the third-largest share by the end of the projected timeline. The segment’s development is highly driven by the provision of long-term bronchodilation, enhancing lung function, diminishing breathlessness, and boosting the overall quality of life. As per an article published by the GOLD COPD Organization in November 2024, ICS treatment is combined with LABA for more than 2 exacerbations of COPD every year, followed by blood eosinophils of over 300 cells, and a record of concomitant asthma.
Our in-depth analysis of the market includes the following segments:
|
Segment |
Subsegments |
|
Route of Administration |
|
|
Drug Type |
|
|
Application |
|
|
Distribution Channel |
|
|
Product Type |
|
|
End user |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Long-acting Beta-agonists Market - Regional Analysis
North America Market Insights
North America in the long-acting beta-agonists market is projected to garner the largest share of 40.3% by the end of 2035. The market’s exposure in the overall region is highly attributed to a surge in respiratory disorders, an increase in federal investment for chronic illness management, and suitable reimbursement policies. As per the 2023 CSM Government data report, healthcare expenditure in the U.S. has reached USD 4.9 trillion, denoting a surge by 7.5% from 4.6% back in 2022. Additionally, the healthcare industry’s economic share in the same year was 17.6%, a slight increase from 17.4% in 2022, thereby making it suitable for the overall market’s development.
U.S. market is growing significantly, owing to effective transformation in the Medicare policy, an upsurge in asthma and COPD cases, and the increased usability of advanced inhalers. Besides, as per an article published by NLM in February 2025, Medicare Part D formularies with clinical treatment approaches include low-dosage inhaled corticosteroid (ICS) treatment, due to which there has been a reduction in hospitalizations by 65% in comparison to traditional treatment. Besides, based on the GINA guideline suggestions, 55% of admitted patients were readily on a reliever therapy, thus denoting a positive impact on the overall market.
Canada market is also growing due to the presence of universal drug coverage policies, robust emphasis on affordability, significant healthcare expenditure, a surge in hospitalization, and the integration of national pharma care approaches. As per a data report published by Health Canada in 2024, the governmental organization is set to boost the public healthcare system, with the government of Canada investing more than USD 200 billion for over 10 years. This includes additional funding provision, such as USD 5.4 billion for the Aging with Dignity bilateral deal, as well as USD 1.7 billion for enhancing personal support workers, thereby suitable for the market’s upliftment.
Wastewater Activity Level Resulting in Respiratory Diseases in America (2025)
|
Countries |
Prevalence |
|
Oregon |
25 |
|
Colorado |
21 |
|
Texas |
20 |
|
Wisconsin |
22 |
|
Illinois |
63 |
|
Indiana |
10 |
|
Ohio |
39 |
|
Pennsylvania |
24 |
Source: CDC
APAC Market Insights
Asia Pacific in the long-acting beta-agonists (LABA) market is expected to emerge as the fastest-growing region during the projected duration. The market’s development in the region is highly subject to an expansion in healthcare facilities, urbanization-specific respiratory illness incidence, and the growing incorporation of fixed-dose LABA/ICS combinations. Different countries in the region are readily making investments in respiratory care through local manufacturing incentives, public procurement programs, and health insurance extension. Besides, the aspects of tender-specific procurement, digitalized health implementation, and localized manufacturing are considered notable market enablers.
The long-acting beta-agonists market in China is gaining increased traction, owing to the existence of government spending for LABA treatments, an increase in patients severely diagnosed with moderate-to-severe COPD, and the presence of the Healthy China 2030 program to encourage early diagnosis and the utilization of digitalized inhalers. As stated in a data report published by the National Bureau of Statistics of China, the national government revenue of the country has been 203,649 yuan in 2022 and 216,795 yuan in 2023, which deliberately contributes to the market’s upliftment.
The market in India is also developing due to the aspect of high price-sensitivity, which is fueled by a surge in the middle-class population, an increase in the awareness of respiratory health, and the presence of government-based strategies, such as the Ayushman Bharat scheme, to offer health coverage. According to a data report published by the NHA in 2023, the Ayushman Bharat scheme caters to 12 crore (120 million) families, which is over 59.7 crore (597 million) individuals, with eligibility criteria, healthcare coverage of almost Rs. 5 Lakhs (USD 5,631.8) per family every year, which denotes a huge growth opportunity for the overall market in the country.
2023 Government Expenditure in Asia
|
Countries |
% of GDP |
|
Australia |
37.2 |
|
China |
33.1 |
|
India |
29.1 |
|
Indonesia |
16.6 |
|
Japan |
41.1 |
|
Malaysia |
24.1 |
Source: International Monetary Fund
Europe Market Insights
Europe market is predicted to steadily grow by the end of the forecast timeline. The market’s upliftment in the region is highly driven by a rise in the aging population, along with strategies to highlight chronic disease management. Besides, the region’s third-leading cause of mortality is continuously fueling the requirement for LABA treatments. In addition, the Europe Medicines Agency has effectively modernized the clearance for LABA/ICS combinations that tend to promote therapeutic innovation and market entry. Meanwhile, the incorporation of advanced inhalers in chronic disease care has readily prioritized the biosimilar LABA, which positively impacts the market in the region.
The long-acting beta-agonists (LABA) market in Germany is gaining increased exposure, owing to the aspect of increased spending on LABA treatment options. In addition, the country’s statutory health insurance initiated the payment for the majority of LABA/ICS combinations, with complete reimbursement through structured care program services for rare respiratory illnesses. Besides, as per an article published by NLM in June 2025, 6.4% of the population aged more than 40 years have been diagnosed with COPD, while recurrent episodes of exacerbations cater to almost 25% of the reduction in lung functions among patients with the condition.
The market in France is also growing due to the existence of the Haute Autorité de Santé (HAS) that effectively ensures wide-ranging coverage for essential medicines. Besides, the market’s exposure in the country is also driven by government support for long-lasting chronic disease management programs, an increase in health and medical spending, and specific provisions for respiratory drugs. As per the May 2024 Euro Health Observatory article, the presence of health spending in the country accounts for USD 5,740, which represents 12.2% of the gross domestic product (GDP). In addition, the public expenditure constitutes 79% of the overall health spending, thereby suitable for the market’s growth.
Key Long-acting Beta-agonists Market Players:
- GlaxoSmithKline plc (GSK)
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- AstraZeneca plc
- Boehringer Ingelheim
- Novartis AG
- Mylan N.V. (Viatris Inc.)
- Teva Pharmaceutical Industries
- Chiesi Farmaceutici S.p.A.
- Cipla Ltd.
- Sun Pharmaceutical Industries Ltd.
- Astellas Pharma Inc.
- Takeda Pharmaceutical Company
- Zambon S.p.A.
- Orion Corporation
- Glenmark Pharmaceuticals
- SK Bioscience Co., Ltd.
- Samsung Biologics
- Lupin Limited
- CSL Limited (Seqirus)
- Bio-pharma Sdn Bhd
- Neopharma
The worldwide market is extremely competitive with the presence of both regional and multinational organizations. Notable firms, including Novartis, GSK, Boehringer Ingelheim, and AstraZeneca, are readily controlling the overall market by branded segment share through combination therapies and long-acting inhalers. Besides, firms from South Korea and India are increasingly gaining attention, owing to affordable generic alternatives as well as API exports, particularly in developing nations. Meanwhile, strategies, including Mylan’s successful merger into Viatris, Samsung Biologics’ partnerships, and Cipla’s EU expansion, are surging competition in the market.
Here is a list of key players operating in the market:
Recent Developments
- In June 2025, Theravance Biopharma, Inc., notified that it has effectively entered into a strategic agreement to purchase its remaining royalty interest of Trelegy Ellipta to GSK for USD 225 million to pool in with GSK’s LABA assets.
- In September 2024, Sanofi declared that the U.S. FDA has readily approved Dupixent as an additional maintenance treatment for adults with inadequately controlled COPD, as well as an eosinophilic phenotype.
- In March 2024, AstraZeneca Pharma India Limited and Mankind Pharma Limited entered into a tactical deal for the outstanding distribution of formoterol fumarate dihydrate and budesonide, which is a combination of ICS and LABA, in India.
- Report ID: 3996
- Published Date: Sep 30, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Long-acting Beta-agonists Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




